Daniel Portnoy

Distinguished Professor of Biochemistry, Biophysics and Structural Biology*
*And Affiliate, Division of Immunology & Pathogenesis
Research Interests
Intracellular pathogens are responsible for an enormous amount of worldwide morbidity and mortality and the development of vaccines and therapeutics to treat diseases caused by these pathogens continues to represent one of the biggest challenges facing the international biomedical community. By virtue of their intracellular niche, these pathogens avoid extracellular immune defense mechanisms, and consequently, vaccine strategies that target the production of antibodies have been largely ineffective. The Portnoy lab tackles a wide range of problems related to the pathogenesis and host response to intracellular pathogens with the goal of developing vaccines and therapeutics. Specifically, the lab works on Listeria monocytogenes, a facultative intracellular food-borne bacterial pathogen that is an outstanding model system with which to dissect basic aspects of host-pathogen interactions.
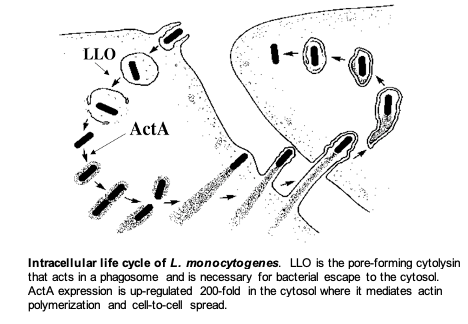
Specifically, the lab is focused on the interaction of the facultative intracellular bacterial pathogen Listeria monocytogenes and its mammalian host. This fascinating microorganism is able to enter cells, escape from a phagosome, circumvent autophagy, avoid cell death pathways, and grow rapidly in the cytosol. By exploiting a host system of actin-based motility, the bacteria move through the cytosol to the cell membrane and into pseudopod-like projections (listeriopods) that are ingested by neighboring cells. This mechanism allows pathogens to spread from one cell to another without ever leaving the host cytoplasm thereby avoiding the immune response. Current research covers many topics including regulation of virulence transcriptional and translational regulation, the cell biology of infection, innate immune responses, acquired immunity, and vaccine development to both infectious diseases and cancer.
Current Projects
Characterization of the essential virulence factor LLO. The L. monocytogenes pore-forming cytolysin, Listeriolysin O (LLO) is an essential determinant of pathogenesis that mediates escape of the bacteria from host phagosomes allowing the bacteria to grow in the cytosol. Although LLO is related to dozens of other members of this family of cytolysins, LLO is the only one that is required by an intracellular pathogen. Accordingly, LLO has evolved numerous properties that allow it to specifically act within the host cell without causing cell death. We are currently studying the regulation of LLO synthesis and the cell biology of its trafficking in cells. We recently discovered that LLO synthesized by cytosolic bacteria forms pores in the cytoplasmic membrane but prevents killing the host cell by mediating endocytosis and targeting LLO for degradation. Mutants lacking this activity are 10,000-fold less virulent. Most recently, we have uncovered a mechanism that controls the translation of LLO so that it is synthesized and secreted during the starvation environment in a phagosome, but much less so during growth in the host cell cytosol.
Regulation of virulence gene expression. L. monocytogenes lives a biphasic lifestyle that includes growth in the environment, including food, and infection of warm-blooded animals, including humans. Most determinants of pathogenesis are controlled by PrfA, the master virulence transcriptional regulator. We found that intracellular bacteria detect the host environment by responding to the redox state of the host cell and up-regulating the synthesis of glutathione, which is the allosteric activator of PrfA. We recently discovered that bacterial detection and detoxification of methylglyoxal, a toxic electrophilic species produced by both bacterial and host cells, leads to up-regulation of glutathione.
Interaction of L. monocytogenes with the innate immune system. We have three new projects that relate bacterial metabolism to host innate immunity.
(1) We discovered that the bacteria secrete c-di-AMP, a conserved signaling molecule that binds to and activates STING, a critical hub for the detection of microorganisms and tumors. We are currently studying the basic microbiology of c-di-AMP and its role during pathogenesis. Most recently, we have begun to explore how activation of STING can lead to trafficking from the intestine to distal sites including the brain and placenta.
(2) Humans possess a particular type of T-cell (gamma delta T- cell) that is activated by an intermediate of bacterial isoprenoid biosynthesis. Some bacteria synthesize this intermediate while others use the same pathway as humans. L. monocytogenes is one of the very few bacteria that use both pathways. We are currently seeking to understand why L. monocytogenes has both pathways and determine the role of each during infection.
(3) Humans and mice have T-cells called MAIT cells that are stimulated by bacterial metabolic intermediates of riboflavin biosynthesis. L. monocytogenes is a riboflavin auxotroph and is not predicted to stimulate MAIT cells. We are current examining if MAIT cells respond to L. monocytogenes infection and if these T cells play a role during L. monocytogenes infection and immunity.
Selected Publications
Chevée V, Hullahalli K, Dailey KG, Güereca L, Zhang C, Waldor MK, Portnoy DA. Temporal and spatial dynamics of Listeria monocytogenes central nervous system infection in mice. Proc Natl Acad Sci USA 2024 121 (17) e2320311121. (2024)
Garcia-Castillo, J, Fernandez S, Campbell T, Williams J, Gonzales-Ventura D, Ybarra J, Flores-Hernandez N, Wells E, Portnoy DA, DuPage M. Cellular mechanisms underlying beneficial versus detrimental effects of bacterial antitumor immunotherapy. bioRxiv. doi: https://doi.org/10.1101.2024.02.15.580555. (2024)
Berude, JC, Kennouche P, Reniere ML, Portnoy DA. Listeria monocytogenes utilizes glutathione and limited inorganic sulfur compounds as a source of essential L-cysteine. Infect Immun. 2024 Jan 30:e0042223. (2024)
Feng Y, Chang SK, Portnoy DA. The major role of Listeria monocytogenes folic acid metabolism during infection is the generation of N-formylmethionine. mBio. 11 Sep 2023: e0107423. Editor's Pick. (2023)
Morrison HM, Craft J, Rivera-Lugo R, Johnson JR, Golovkine GR, Bell SL, Dodd CE, Van Dis E, Beatty WL, Margolis SR, Repasy T, Shaker I, Lee AY, Vance RE, Stanley SA, Watson RO, Krogan NJ, Portnoy DA, Penn BH, Cox JS. Deficiency in Galectin-3, -8, and -9 impairs immunity to chronic Mycobacterium tuberculosis infection but not acute infection with multiple intracellular pathogens. PLoS Pathog. 2023 Jun 23;19(6): e1011088. (2023)
Rivera-Lugo R, Huang S, Lee F, Méheust R, Iavarone AT, Sidebottom AM, Oldfield E, Portnoy DA, Light SH. Distinct energy-coupling factor transporter subunits enable flavin acquisition and extracytosolic trafficking for extracellular electron transfer in Listeria monocytogenes. mBio. 2023 Feb 28;14(1):e0308522. (2023)
Zhang Y, Anaya-Sanchez A, Portnoy DA. para-Aminobenzoic acid biosynthesis is required for Listera monocytogenes growth and pathogenesis. Infect Immun. 2022 Nov 17:90(11):e0020722. (2022)
Rivera-Lugo R, Deng D, Anaya-Sanchez A, Tejedor-Sanz S, Tang E, Reyes Ruiz VM, Smith HB, Titov DV, Sauer JD, Skaar EP, Ajo-Franklin CM, Portnoy DA, Light SH. Listeria monocytogenes requires cellular respiration for NAD+ regeneration and pathogenesis. Elife. 2022 Apr 5;11:e75424. (2022)
Rivera-Lugo R, Light SH, Garelis NE, Portnoy DA. RibU is an essential determinant of Listeria pathogenesis that mediates acquisition of FMN and FAD during intracellular growth. Proc Natl Acad Sci USA. 2022 Mar 29;119(13):e2122173119. (2022)
Anaya Sanchez A, Feng Y, Berude J, Portnoy DA. Detoxification of methylglyoxal by the glyoxalase system is required for glutathione availability and virulence activation in Listeria monocytogenes. PLOS Pathogens. 2021 Aug 18:17(8):e1009819. (2021)
Ji DX, Witt KC, Kotov DI, Margolis SR, Louie A, Chevée V, Chen KJ, Gaidt MM, Dhaliwal HS, Lee AY, Nishimura SL, Zamboni DS, Kramnik I, Portnoy DA, Darwin KH, Vance RE. Role of the transcriptional regulator SP140 in resistance to bacterial infections via reporession of type I interferons. Elife. 2021 Jun 21;10:e67290. (2021)
Louie A, Bhandula V, Portnoy DA. Secretion of c-di-AMP by Listeria monocytogenes leads to a STING-dependent antibacterial response during enterocolitis. Infect Immun. 2020 Oct 5:IAI.00407-20. (2020)
Peterson BN, Portman JL, Feng Y, Wang J, Portnoy DA. Secondary structure of the mRNA encoding listeriolysin O is essential to establish the replicative niche of L. monocytogenes. Proc Natl Acad Sci USA. 2020 Sep 22;117(38):23774-23781. (2020)
Peterson BN, Young MKM, Luo S, Wang J, Whiteley AT, Woodward JJ, Tong L, Wang JD, Portnoy DA. (p)ppGpp and c-di-AMP homeostasis is controlled by CbpB in Listeria monocytogenes. mBio. 2020 Aug 25;11(4):e01625-20. (2020)
Nguyen BN, Chavez-Arroyo A, Louie A, Cheng MI, Krasilnikov M, Portnoy DA. TLR2 and endosomal TLR-mediated secretion of IL-10 and immune suppression in response to phagosome-confined Listeria monocytogenes. PLoS Pathog. 2020 Jul 7;16(7):e1008622. (2020)
Lee ED, Navas KI, Portnoy DA. The nonmevalonate pathway of isoprenoid biosynthesis supports anaerobic growth of Listeria monocytogenes. Infect Immun. 2020 Jan 22;88(2):e00788-19. (2020)
Nguyen BN, Portnoy DA. An inducible Cre-lox system to analyze the role of LLO in Listeria monocytogenes pathogenesis. Toxins. 2020 Jan 7;12(1):38. (2020)
Last Updated 2024-08-26