MCB TRANSCRIPT

Structural Biology: How Proteins Give Membranes Form and Function
MCB professor James Hurley is fascinated by cell membranes, which are dwarfed by the vastness of a cell but are hugely influential. “Proteins and lipids come together to give membranes their shape and function,” he says. “Membranes delimit everything in eukaryotic cells, and also send signals and create energy.”

To find out how membranes move and change, Hurley takes a close look at individual membrane proteins with techniques like X-ray crystallography, which reveals details down to the atomic level, and cryo-electron microscopy, which catches molecules in action.
One protein complex he’s looking at is ESCRT, which forms vesicles that HIV-1 likely hijacks to propagate itself. “If we can block that, there will be no infection,” Hurley says.
To identify targets for HIV drugs, he wants a detailed picture of the ESCRT proteins. His goal is to make a synthetic membrane with both ESCRT and a common HIV coat protein called Gag, which will let him watch the vesicle-forming complex release Gag. “Then we will have an amazing ability to dissect the process,” he says. “We will be able to pick it apart atom by atom.” The system will also let him test the hypothesis that HIV hijacks ESCRT into serving the virus instead of the host.
Hurley also studies autophagy, which literally means “to eat oneself.” Autophagy entails growing a membrane around part of the internal contents of a cell and then fusing the resulting autophagosome with a lysosome, which recycles the autophagosome contents. This cellular self-cannibalism may sound bizarre but is crucial to keeping us healthy.
“It’s the only way to get rid of damaged cell parts like leaky mitochondria,” Hurley explains. As we age, autophagy diminishes and cellular damage accumulates. For example, damaged mitochondria are implicated in Parkinson’s disease. “They’re like having little nuclear reactors in the brain,” he says.
Despite their importance, we don’t know much about how autophagosomes are made. We know many of the proteins that are involved but not exactly how they work together, so Hurley is studying the basic mechanism of autophagosome formation. “How is it initiated, how is it shaped, and how does it grow?” he asks. So far, he has solved the structure of the Atg1 complex, which initiates the process of autophagy and may tether the first membrane vesicles that become part of the autophagosome.
One of the things that drew him to UC Berkeley was the chance to work with so many like-minded cell biologists. “It’s very important for biophysical work to be grounded in cell biology, and this is one of the most collaborative research environments in the world,” Hurley says. “I love it — it’s very stimulating.”
In recognition of his work, Hurley has recently received the 2014 Hans Neurath Award from the Protein Society.
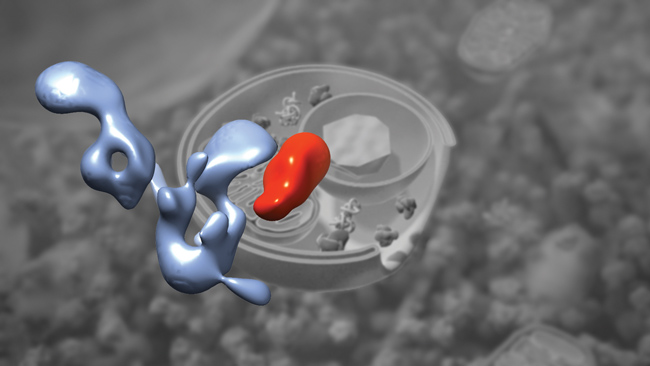
overlaid on a drawing of a growing autophagosome.




