 |
| Left to right: MCB Professor James Hurley and MCB Assistant Professor Roberto Zoncu. |
A team of researchers from the labs of MCB Professor and Judy C. Webb Chair James Hurley and MCB Assistant Professor Roberto Zoncu report the discovery of a novel checkpoint in cell growth signaling: the Lysosomal Folliculin Complex (LFC). The LFC controls activation of the master regulator of cell growth and mechanistic Target of Rapamycin Complex 1 (mTORC1), a core growth signaling pathway that is frequently mutated in cancer, diabetes, and neurodegeneration.
Published online today, their paper reveals a high-resolution structure of the LFC achieved via Cryo-Electron Microscopy (Cryo-EM), representing the first full structure of the tumor suppressor Folliculin (FLCN) with its interaction partner Folliculin Interacting Partner 2 (FNIP2), along with the mTORC1 regulators the Rag GTPase and Ragulator complex.
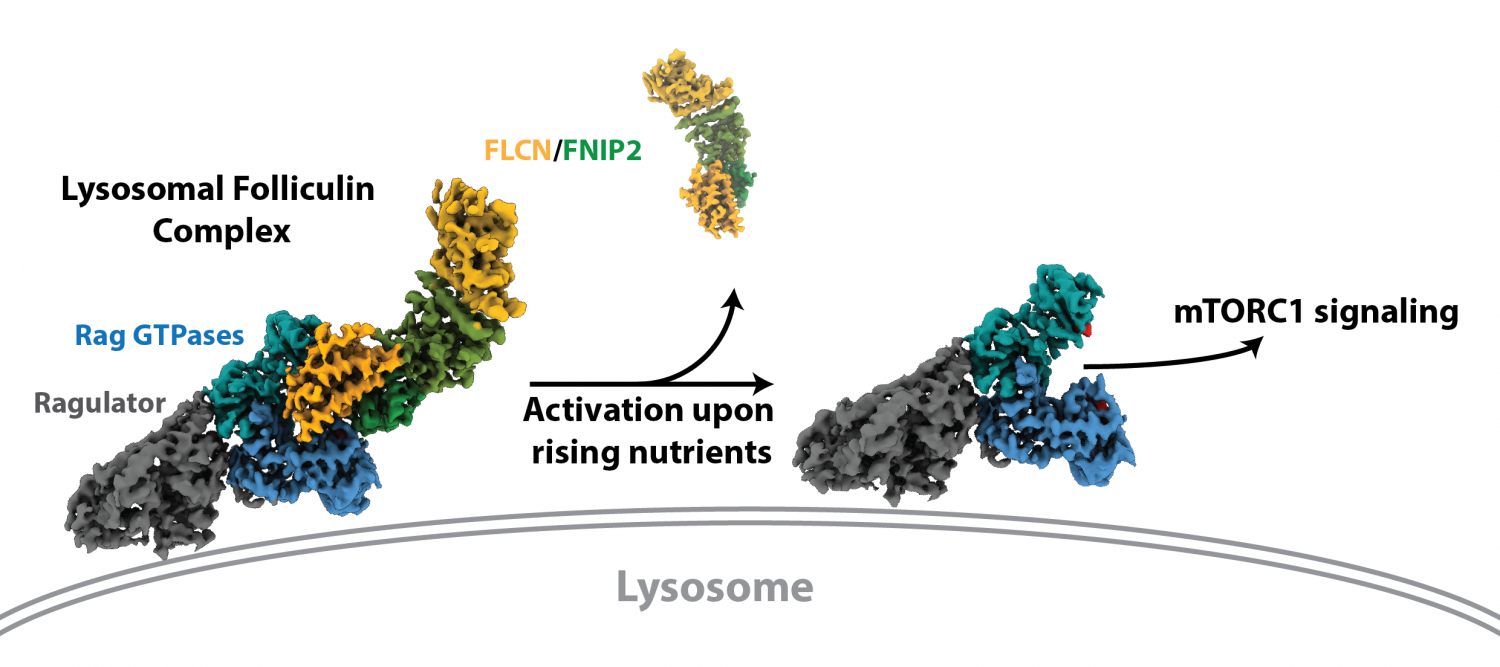 When cellular nutrient levels are high enough to support growth, mTORC1 is recruited to the surface of the lysosome, a catabolic organelle that is also a metabolic signaling hub. Once at the lysosome, mTORC1 is activated to turn on cellular growth pathways, including the production of proteins, lipids, and energy. The proteins that control mTORC1 recruitment to the lysosome play key roles in regulating cellular growth and averting disease states. These include the Rag GTPase protein complex, which recruits mTORC1 to the lysosome when converted to an active nucleotide-binding state. This process requires the FLCN/FNIP2 complex.
When cellular nutrient levels are high enough to support growth, mTORC1 is recruited to the surface of the lysosome, a catabolic organelle that is also a metabolic signaling hub. Once at the lysosome, mTORC1 is activated to turn on cellular growth pathways, including the production of proteins, lipids, and energy. The proteins that control mTORC1 recruitment to the lysosome play key roles in regulating cellular growth and averting disease states. These include the Rag GTPase protein complex, which recruits mTORC1 to the lysosome when converted to an active nucleotide-binding state. This process requires the FLCN/FNIP2 complex.
The researchers discovered the LFC assembles at the lysosome surface when nutrient levels are low, and allows both the Rag GTPases and FLCN/FNIP2 to mutually inhibit each another to prevent mTORC1 activation until nutrients rise and the complex disassembles.
In summary this work, which was co-led by MCB graduate student Rosalie Lawrence (Zoncu lab) and postdoctoral fellow Simon Fromm (Hurley lab) identifies a novel checkpoint in cellular growth signaling and uncovers a new layer of regulation of cell growth that may be impaired in human disease, and provides a high resolution structural map of the LFC.
Read the online Science paper here.