Michel DuPage

Assistant Professor of Immunology and Molecular Medicine
Lab Homepage: https://mcb.berkeley.edu/labs/dupage/Research Interests
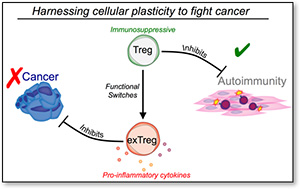
The success of multiple forms of immune-based cancer therapies has revolutionized the way we approach the treatment of cancer. However, it is already apparent that our current arsenal of new drugs will not benefit all patients, and many drugs generate severe side effects. Research in my laboratory aims to unleash the full potential of our immune system to fight cancer by understanding the underlying mechanisms that enable beneficial immune responses against tumors while minimizing the detrimental responses against healthy tissues. We are particularly interested in investigating regulatory T cells (Tregs), a specialized type of T cell with immunosuppressive activity. In cancers, Tregs heavily infiltrate tumors, supporting cancer outgrowth by dampening anti-tumor immune responses. In contrast, in autoimmune diseases, Tregs can be reprogrammed to promote inflammation, exacerbating disease. We are fascinated by this natural plasticity in T cell function and we hope to exploit it for therapeutic purposes by identifying and targeting the regulatory circuits inside T cells that act as gatekeepers between different functional states.
Current Projects
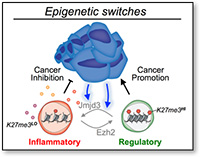
 Epigenetic switches in Treg function.
Epigenetic switches in Treg function.
My work has identified the epigenetic enzyme EZH2, a histone H3K27 methyltransferase, to be a critical mediator of Treg stability and function in activated Tregs. Strikingly, disrupting EZH2 function in Tregs endows mice with strong resistance to the development of multiple cancers. Conversely, mice with a Treg-specific deficiency of the H3K27me3 demethylase JMJD3, have an enlarged population of stable Tregs and tumors grow faster. Ongoing research is aimed at understanding how these epigenetic modifications reprogram intratumoral Tregs and shape the anti-tumor immune response. We are also very interested in the role of other histone modifications in Treg functions and are developing new genetic tools to define the contributions of additional modifications.
 Genetically engineered mouse models of cancer.
Genetically engineered mouse models of cancer.
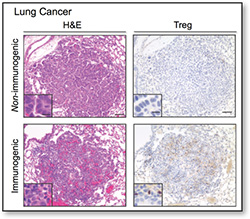
To understand how the immune system recognizes and interacts with cancers, it is essential that our experimental models accurately recapitulate the features of the human disease. Genetically engineered mouse (GEM) models of cancer provide the most faithful recapitulation of the human disease in mice because they harbor identical genetic and histopathologic features and arise de novo from the transformation of normal cells into cancers within their native environments. 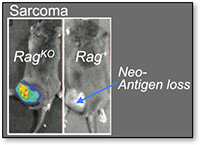 I have pioneered the use of several GEM models to investigate immune response to cancer. This work has revealed the context-dependent nature of immune-tumor interactions, but most importantly, it was at the forefront of a transformation in thinking about the importance of tumor-specific neo-antigens in cancer immunotherapy. Our lab will continue to pioneer and utilize the most sophisticated models of cancer to define the rules for how cells of the immune system interact with cancers.
I have pioneered the use of several GEM models to investigate immune response to cancer. This work has revealed the context-dependent nature of immune-tumor interactions, but most importantly, it was at the forefront of a transformation in thinking about the importance of tumor-specific neo-antigens in cancer immunotherapy. Our lab will continue to pioneer and utilize the most sophisticated models of cancer to define the rules for how cells of the immune system interact with cancers.
Selected Publications
DuPage M, Bluestone JA. Harnessing the plasticity of CD4+ T cells to treat immune-mediated disease. Nat Rev Immunol (2016) 16: 149-163.
DuPage M, Chopra G, Quiros J, Rosenthal WL, Morar MM, Holohan D, Zhang R, Turka L, Marson A, Bluestone JA. The chromatin-modifying enzyme Ezh2 is critical for the maintenance of regulatory T cell identity after activation. Immunity (2015) 42: 227-238.
Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM, Townamchai N, Gerriets VA, Rathmell JC, Sharpe AH, Bluestone JA, Turka LA. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol (2015) 16: 188-96.
Joshi NS, Akama-Garren EH, Lu Y, Lee DY, Chang GP, Li A, DuPage M, Tammela T, Kerper NR, Farago AF, Robbins R, Crowley DM, Bronson RT, Jacks T. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity (2015) 43: 579-590.
DuPage M, Jacks T. Genetically engineered mouse models of cancer reveal new insights about the antitumor immune response. Curr Opin Immunol (2013) 25: 192-199.
DuPage M, Mazumdar C, Schmidt LM, Cheung AF, Jacks T. Expression of tumour-specific antigens underlies cancer immunoediting. Nature (2012) 482: 405-409.
DuPage M, Cheung AF, Mazumdar C, Winslow MM, Bronson R, Schmidt LM, Crowley D, Chen J, Jacks T. Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression. Cancer Cell (2011) 19: 72-85.
DuPage M*, Dooley AL*, Jacks T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc (2009) 4: 1064-1072.
Cheung AF, DuPage M, Dong HK, Chen J, Jacks T. Regulated expression of a tumor-associated antigen reveals multiple levels of T-cell tolerance in a mouse model of lung cancer. Cancer Res (2008) 68: 9459-68.
Last Updated 2017-06-23