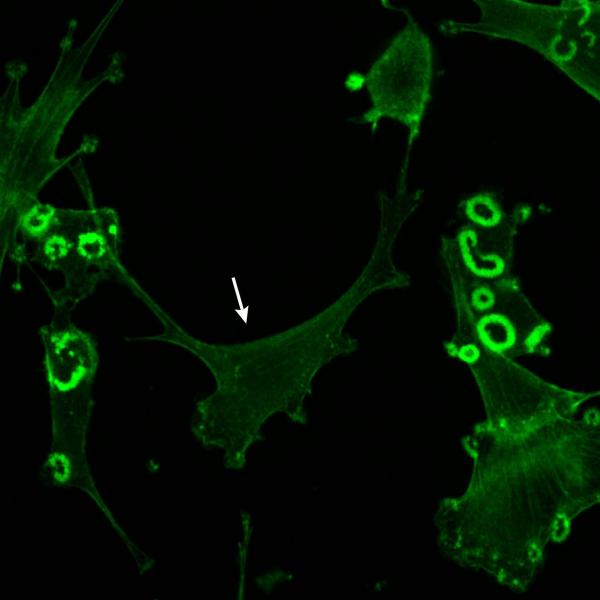
Src-induced podosomes need Rho
The Martin lab has found that active Rho (Rho[GTP]) is localized to podosomes in oncogenically transformed cells. These matrix-digesting, actin-rich, short-lived protrusions help osteoclasts to eat bone and migratory and cancer cells to chew their way through matrix. Their function in Src-transformed cells has now been found to rely on Rho activity.
Rho is known to promote the formation of focal adhesions and actin stress fibers, so the disappearance of stress fibers after transformation with oncogenic Src was presumed to reflect downregulation of Rho. But the Martin lab has found that levels of Rho[GTP] are not decreased in Src-transformed cells. Instead, focal adhesions and stress fibers may be lost because of some block in signaling downstream of Rho and the loss in cell-matrix adhesion induced by Src. Rho may be locally activated by Src at podosomes, or Rho[GTP] might be generated elsewhere and then move to the podosomes.
Inhibition of Rho disrupted localization of several proteins to podosomes, and almost completely abolished the secretion of matrix-digesting enzymes. Thus Rho is necessary to form not only focal adhesions and stress fibers in normal cells but also the more dynamic and transient podosomes characteristic of transformed and tumor cells. The relevant Rho effectors are not known, but Rho may work by recruiting active Src to podosomes or by reorganizing the actin core in these structures. Several of the many signaling proteins in podosomes may, in turn, regulate the localization and activation of Rho.
Berdeaux, R., B. Díaz, L. Kim, and G. S. Martin. 2004. Active Rho is localized to podosomes induced by oncogenic Src and is required for their assembly and function. J Cell Biol 166:317-323.
Photo courtesy of Professor G. Steven Martin.