Dirk Hockemeyer

C.H. and Annie Li Chair in Molecular Biology of Diseases and Professor of Cell Biology, Development and Physiology*
*and
Research Interests
Our goal is to shed light on the key functions of telomeres and telomerase in tissue homeostasis, tumorigenesis and aging. Telomeres are the repetitive DNA sequences at the end of linear eukaryotic chromosomes that allow a cell to distinguish the natural chromosome end from aberrant DNA breaks. Telomeric DNA repeats can be generated de novo by the enzyme telomerase thereby providing a compensatory mechanism that counteracts terminal sequence loss caused by the end replication problem. 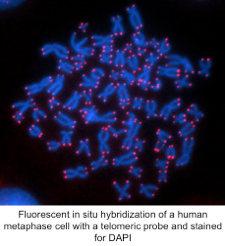 As a result, telomeres and telomerase are essential to genomic integrity and their disruption is associated with cancer and aging. The use of genetic mouse models has been a powerful way to gain insight into the fundamental mechanisms of how the telomere evades recognition by the DNA-damage machinery, the consequences of telomerase loss, and how the single stranded telomeric overhang is established. However, telomere shortening naturally occurs only in human somatic cells, but not in mouse cells. This telomere shortening, which functions as a tumor suppressor mechanism by limiting the replicative potential of human cells, is the result of selective silencing of telomerase expression in human cells upon their differentiation. Notably, this process is reversed and telomerase reactivated in about 90% of all human tumors after which telomerase expression becomes essential for their proliferation.
As a result, telomeres and telomerase are essential to genomic integrity and their disruption is associated with cancer and aging. The use of genetic mouse models has been a powerful way to gain insight into the fundamental mechanisms of how the telomere evades recognition by the DNA-damage machinery, the consequences of telomerase loss, and how the single stranded telomeric overhang is established. However, telomere shortening naturally occurs only in human somatic cells, but not in mouse cells. This telomere shortening, which functions as a tumor suppressor mechanism by limiting the replicative potential of human cells, is the result of selective silencing of telomerase expression in human cells upon their differentiation. Notably, this process is reversed and telomerase reactivated in about 90% of all human tumors after which telomerase expression becomes essential for their proliferation.
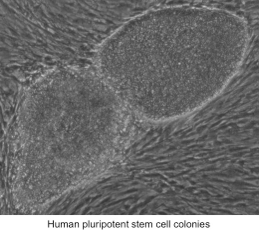 Human pluripotent stem cells (hPSC) are an ideal model system to study telomerase regulation, as they are telomerase-positive, but can be rapidly differentiated into telomerase-negative cells. Moreover, the reverse process of telomerase reactivation can be studied during the process of reprogramming somatic cells into induced pluripotent stem cells (iPSCs). Owing to their constitutive telomerase expression, PSCs are immortal and can be expanded indefinitely in culture. However, in contrast to other model systems such as tumor cells, they have functional DNA surveillance and cell cycle checkpoints and therefore are ideal for studying the effects of telomere shortening and dysfunction on these pathways.
Human pluripotent stem cells (hPSC) are an ideal model system to study telomerase regulation, as they are telomerase-positive, but can be rapidly differentiated into telomerase-negative cells. Moreover, the reverse process of telomerase reactivation can be studied during the process of reprogramming somatic cells into induced pluripotent stem cells (iPSCs). Owing to their constitutive telomerase expression, PSCs are immortal and can be expanded indefinitely in culture. However, in contrast to other model systems such as tumor cells, they have functional DNA surveillance and cell cycle checkpoints and therefore are ideal for studying the effects of telomere shortening and dysfunction on these pathways.
Over the last few years we have developed robust protocols to derive and genetically modify human PSCs.Prior to this, primary human cells were resilient to conventional homologous recombination making gene targeting extremely inefficient and time consuming.
Due to these technical advancements we can now overexpress or silence genes, correct disease-causing mutations, and engineer reporter genes in human PSCs. In my laboratory, we will use these gene targeting strategies to study the biology of human telomeres to address:
- What is the mechanism of telomerase regulation in human stem cells, upon their differentiation and during tumor formation?
- What are the molecular mechanisms underlying the recruitment of telomerase to the telomere in human cells?
- What are the consequences of telomere shortening in stem cells and how does this impact tumor formation?
Selected Publications
Chiba K, Vogan JM, Wu RA, Gill MS, Zhang X, Collins K, Hockemeyer D. (2017) Endogenous TERT N-terminal tagging affects human telomerase function at telomeres in vivo. Molecular and Cell Biology 37(3) e00541-16
Vogan JM, Zhang X, Youmans DT, Regalado SG, Johnson JZ, Hockemeyer D, Collins K. (2016) Minimized human telomerase maintains telomeres and resolves endogenous roles of H/ACA proteins, TCAB1, and Cajal bodies. eLife 5: e18221
Hockemeyer D, Jaenisch R. (2016) Induced Pluripotent Stem Cells Meet Genome Editing. Cell Stem Cell 18(5) 573-586
Blair JD, Bateup H, Hockemeyer D. (2016) Establishment of Genome-edited Human Pluripotent Stem Cell Lines: From Targeting to Isolation. Journal of Visualized Experiments (108): 53583.
Johnson JZ, Hockemeyer D. (2015) Human stem cell-based disease modeling: prospects and challenges. Current Opinion in Cell Biology 37: 84-90
Hockemeyer D, Collins, K. (2015) Control of telomerase action at human telomeres. Nature Structural and Molecular Biology 22(11) 848-852
Chiba K, Johnson JZ, Vogan JM, Wagner T, Boyle JM, Hockemeyer D. (2015) Cancer-associated TERT promoter mutations abrogate telomerase silencing. eLife 4:e07918
Chiba K, Hockemeyer D. (2015) Genome Editing in Human Pluripotent Stem Cells Using Site-Specific Nucleases. Methods in Molecular Biology 1239:267-280
Kabir S, Hockemeyer D, de Lange T. (2014) TALEN Gene Knockouts Reveal No Requirement for the Conserved Human Shelterin Protein Rap1 in Telomere Protection and Length Regulation. Cell Reports. Cell Reports 9(4):1273-80
Sexton AN, Regalado SG, Lai CS, Cost GJ, O'Neil CM, Urnov FD, Gregory PD, Jaenisch R, Collins K, Hockemeyer D. (2014) Genetic and molecular identification of three human TPP1 functions in telomerase action: recruitment, activation, and homeostasis set point regulation. Genes and Development 28(17)
Forster R, Chiba K, Schaeffer L, Regalado SG, Lai C, Gao Qing, Kiana S, Farin HF, Clevers H, Cost GJ, Chan A, Rebar EJ, Urnov FD, Gregory PD, Pachter L, Jaenisch R, Hockemeyer D. (2014) Human intestinal tissue with adult stem cell properties derived from pluripotent stem cells. Cell Stem Cell Reports 2(6):838-52
Forster R, Hockemeyer D. (2014) Genome editing 101: Let's go digital. Nature Methods 11, 248-249
Photo credit: Mark Hanson of Mark Joseph Studios.
Last Updated 2017-06-19