Research
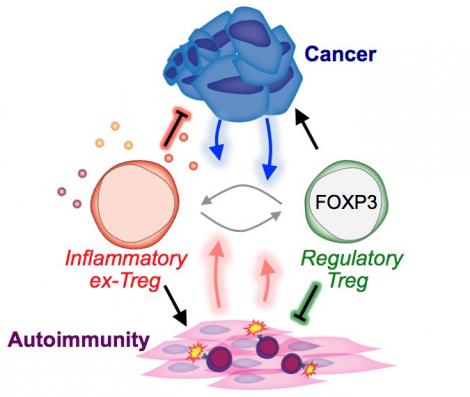 The treatment of cancer has been revolutionized by the development of immune-based therapies designed to boost the number and function of cytotoxic T cells that kill tumor cells. However, it is already apparent that this strategy alone will not benefit all patients, as the majority of cancers generate a highly immunosuppressive microenvironment that shuts down even the most potent T cells. Furthermore, as a consequence of ramping up the immune system, these immune stimulating drugs instigate significant autoimmune toxicity. Our lab is pursuing a radically new strategy to circumvent both challenges by reprogramming the functionality of Regulatory T cells (Tregs) from an immunosuppressive to pro-inflammatory state specifically within the tumor microenvironment. Reprogramming tumor-infiltrating Tregs will be more powerful than other forms of immunotherapy because it can both subvert immune tolerance, by removing immunosuppressive cells from tumors, and directly boost anti-tumor immunity, by converting immunosuppressive cells into immunostimulatory ones. The lab combines sophisticated genetic techniques, genomic analyses, and single cell assays to interrogate immune regulation in the settings of cancer and autoimmunity.
Ongoing research projects in the areas of epigenetics, immunology, and cancer biology include:
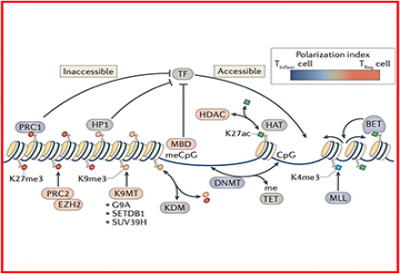
1) Developing new technologies to rapidly assess the role of multiple epigenetic modifications in T cell function.
2) Assessing the effect of anti-cancer drugs targeting epigenetic pathways on the immune system.
3) Determing the parameters governing Treg recruitment and recirculation into tumors and inflammed tissues.
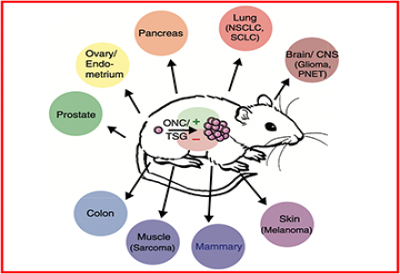
4) Generating sophisticated mouse models of cancer to reveal critical elements of immune-tumor interactions.
5) Interogating the epigenetic properties of effector T cells and Tregs in human cancers.
The treatment of cancer has been revolutionized by the development of immune-based therapies designed to boost the number and function of cytotoxic T cells that kill tumor cells. However, it is already apparent that this strategy alone will not benefit all patients, as the majority of cancers generate a highly immunosuppressive microenvironment that shuts down even the most potent T cells. Furthermore, as a consequence of ramping up the immune system, these immune stimulating drugs instigate significant autoimmune toxicity. Our lab is pursuing a radically new strategy to circumvent both challenges by reprogramming the functionality of Regulatory T cells (Tregs) from an immunosuppressive to pro-inflammatory state specifically within the tumor microenvironment. Reprogramming tumor-infiltrating Tregs will be more powerful than other forms of immunotherapy because it can both subvert immune tolerance, by removing immunosuppressive cells from tumors, and directly boost anti-tumor immunity, by converting immunosuppressive cells into immunostimulatory ones. The lab combines sophisticated genetic techniques, genomic analyses, and single cell assays to interrogate immune regulation in the settings of cancer and autoimmunity.
Ongoing research projects in the areas of epigenetics, immunology, and cancer biology include:
1) Developing new technologies to rapidly assess the role of multiple epigenetic modifications in T cell function.
2) Assessing the effect of anti-cancer drugs targeting epigenetic pathways on the immune system.
3) Determing the parameters governing Treg recruitment and recirculation into tumors and inflammed tissues.
4) Generating sophisticated mouse models of cancer to reveal critical elements of immune-tumor interactions.
5) Interogating the epigenetic properties of effector T cells and Tregs in human cancers.
 The treatment of cancer has been revolutionized by the development of immune-based therapies designed to boost the number and function of cytotoxic T cells that kill tumor cells. However, it is already apparent that this strategy alone will not benefit all patients, as the majority of cancers generate a highly immunosuppressive microenvironment that shuts down even the most potent T cells. Furthermore, as a consequence of ramping up the immune system, these immune stimulating drugs instigate significant autoimmune toxicity. Our lab is pursuing a radically new strategy to circumvent both challenges by reprogramming the functionality of Regulatory T cells (Tregs) from an immunosuppressive to pro-inflammatory state specifically within the tumor microenvironment. Reprogramming tumor-infiltrating Tregs will be more powerful than other forms of immunotherapy because it can both subvert immune tolerance, by removing immunosuppressive cells from tumors, and directly boost anti-tumor immunity, by converting immunosuppressive cells into immunostimulatory ones. The lab combines sophisticated genetic techniques, genomic analyses, and single cell assays to interrogate immune regulation in the settings of cancer and autoimmunity.
Ongoing research projects in the areas of epigenetics, immunology, and cancer biology include:
1) Developing new technologies to rapidly assess the role of multiple epigenetic modifications in T cell function.
2) Assessing the effect of anti-cancer drugs targeting epigenetic pathways on the immune system.
3) Determing the parameters governing Treg recruitment and recirculation into tumors and inflammed tissues.
4) Generating sophisticated mouse models of cancer to reveal critical elements of immune-tumor interactions.
5) Interogating the epigenetic properties of effector T cells and Tregs in human cancers.
The treatment of cancer has been revolutionized by the development of immune-based therapies designed to boost the number and function of cytotoxic T cells that kill tumor cells. However, it is already apparent that this strategy alone will not benefit all patients, as the majority of cancers generate a highly immunosuppressive microenvironment that shuts down even the most potent T cells. Furthermore, as a consequence of ramping up the immune system, these immune stimulating drugs instigate significant autoimmune toxicity. Our lab is pursuing a radically new strategy to circumvent both challenges by reprogramming the functionality of Regulatory T cells (Tregs) from an immunosuppressive to pro-inflammatory state specifically within the tumor microenvironment. Reprogramming tumor-infiltrating Tregs will be more powerful than other forms of immunotherapy because it can both subvert immune tolerance, by removing immunosuppressive cells from tumors, and directly boost anti-tumor immunity, by converting immunosuppressive cells into immunostimulatory ones. The lab combines sophisticated genetic techniques, genomic analyses, and single cell assays to interrogate immune regulation in the settings of cancer and autoimmunity.
Ongoing research projects in the areas of epigenetics, immunology, and cancer biology include:
1) Developing new technologies to rapidly assess the role of multiple epigenetic modifications in T cell function.
2) Assessing the effect of anti-cancer drugs targeting epigenetic pathways on the immune system.
3) Determing the parameters governing Treg recruitment and recirculation into tumors and inflammed tissues.
4) Generating sophisticated mouse models of cancer to reveal critical elements of immune-tumor interactions.
5) Interogating the epigenetic properties of effector T cells and Tregs in human cancers.