Current Projects
- The Role of Central Tolerance in Autoimmunity
- The Role of Programmed Necrosis in Immunity against Virus and Tumor
Research Summaries
T cell self-tolerance is thought to involve central tolerance (negative selection) and peripheral mechanism (e.g. Treg). The role of peripheral tolerance has been convincingly demonstrated by the lethal autoimmune phenotype of Treg-less Foxp3- mice. In contrast, the importance of negative selection in prevention of autoimmunity was still not completely understood. There are several players of apoptosis involved in negative selection:
1) Bim, a BH3-only Bcl-2 family protein is one of them but B6 Bim-/- mice don’t suffer from T cell autoimmunity. In addition, mice with T cell-specific transgenic expression of Bcl-2, which can block all the BH3-only proteins, don’t suffer from autoimmunity.
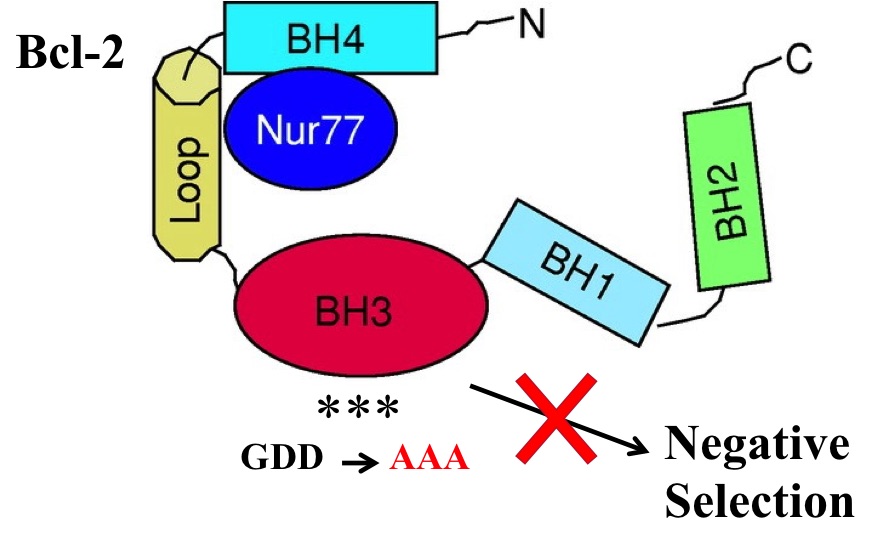
2) Nur77 orphan steroid receptor and its family member Nor-1 have also been implicated in negative selection. Nur77/Nor-1 can translocate to mitochondria and may mediate apoptosis through Bcl-2 by converting Bcl-2 into a pro-apoptotic molecule. We found that T cell specific transgenic (Tg) expression of a mutant Bcl-2 where its potential pro-apoptotic BH3 domain was mutated (Bcl-2-BH3*), results in mice with multi-organ autoimmunity. Aged Bcl-2-BH3* mice in the autoimmune-resistant C57BL/6 background progressively accumulate activated, autoreactive T cells with lymphocytes infiltration in all the organs examined and subsequent morbidity. Antisera from these mice recognize self-proteins with western blot profiles of a given tissue that don’t vary much from one mouse to another.
Current projects are aimed at understanding how Bcl-2-BH3* but not Bcl-2 Tg mice suffer from autoimmunity and to test the idea if these autoreactive T cells can recognize and reject tumor. Successful implementation of these projects should lead to a deeper understanding of the mechanism and role of negative selection in prevention of autoimmunity and its possible utilization in cancer immunotherapy.
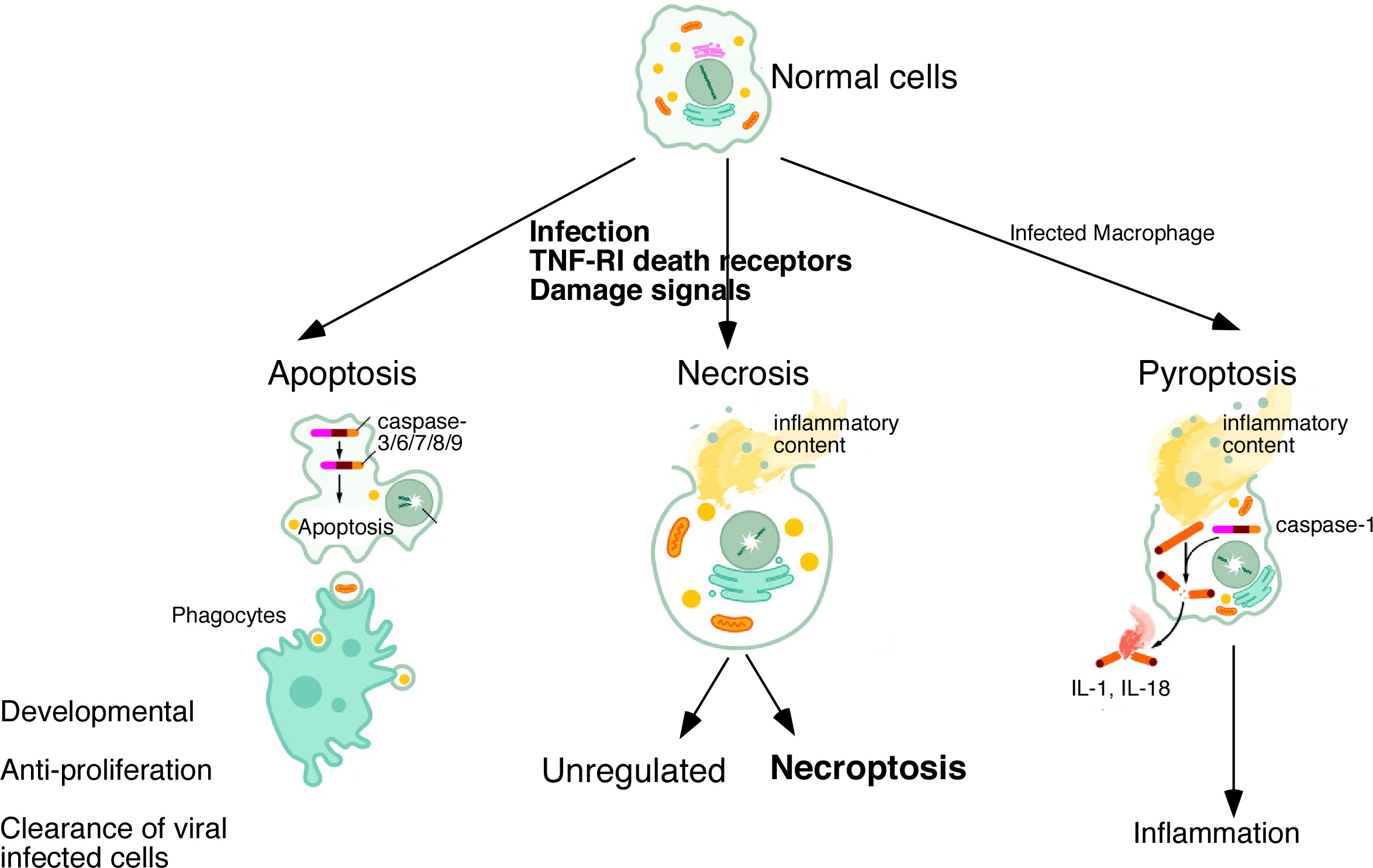
Members of the Tumor Necrosis Factor (TNF) superfamily are involved in diverse biological function, including inflammation, prevention of autoimmunity, cancer, and many others. Indeed, members of this family have become successful targets of drug development for many human diseases. We are interested in the signal transduction events of a subset of the TNF superfamily that can initiate cell death. We have shown that FADD is a universal adapter for all TNF receptor-mediated death. Surprisingly, FADD and caspase-8 are also negative regulators of an alternative form of cell death called necroptosis (programmed necrosis). Loss of FADD leads to resistance to apoptosis but FADD-deficient cells undergo necroptosis instead. Current projects are aimed to understand the physiological role of this "hidden" cell death pathway in infectious diseases and cancer.
updated March 21, 2016
