Laboratory of John G. ForteDepartment of Cell & Molecular Biology, University of California, Berkeley |
Morphological and functional transformation of the
gastric parietal cell between resting and secreting states
|
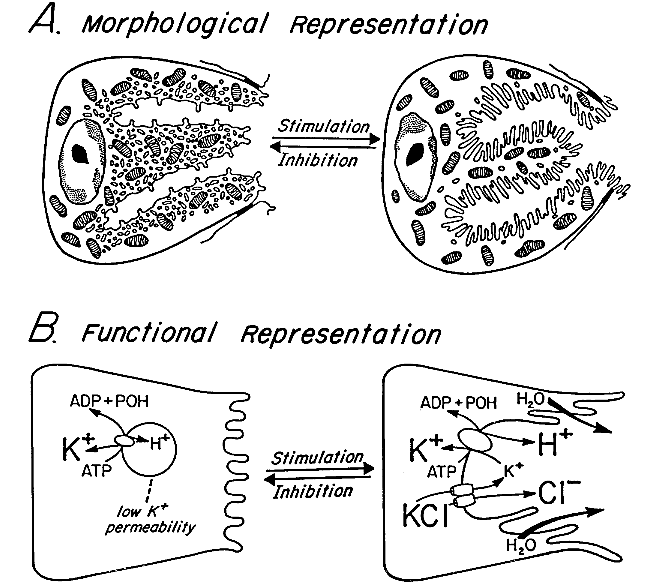
In the parietal cell's non-secreting state, the proton pumps (H,K-ATPase) are mainly in a complex intracellular membrane compartment consisting of many small vesicles having spherical, elongated or flattened shapes and commonly called "tubulovesicles." These membranes lack a potassium ( |
|
|
Upon stimulation, many of the tubulovesicles fuse with the canalicular membrane, moving the proton pumps to a position in which they can actively exchange Protons transported to the canalicular lumen are joined by electrically balancing |
| * |
The process shifts the excess base from parietal cells to the blood, which is better able to handle the excess. A much larger amount of bicarbonate already in the blood (part of the Acid and base are only temporarily separated. When acidic stomach contents empty into the duodenum, bicarbonate, secreted as sodium bicarbonate by the pancreas and duodenal lining, neutralizes HCl to form carbon dioxide, salt, and water: |