Laboratory of John G. ForteDepartment of Cell & Molecular Biology, University of California, Berkeley |
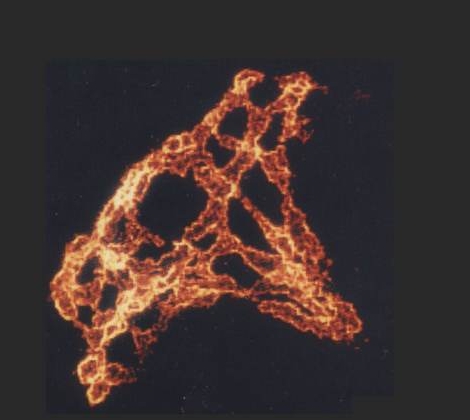
The parietal cell canalicular network, visualized by fluorescent labeling of F-actin and ezrin in isolated rabbit gastric glands |

| 
| 
|
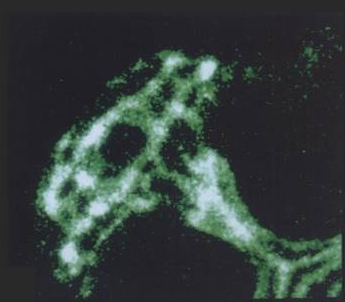
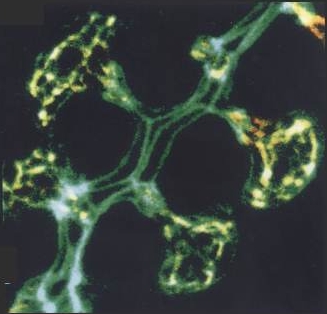
| Ezrin is concentrated at canalicular (apical) membrane of gastric parietal cell, showing extent, and complex branching, of apical membrane invaginated from small apical pore at lower right. (Probed with mouse anti-ezrin & Texas Red-labeled secondary) | F-actin is concentrated along canalicular membrane and extending through cell's apical pore into gland lumen, part of which is seen at lower right. (Probed with FITC-labeled phalloidin) | Lower-power view of gastric gland, with ezrin and F-actin images merged. Colocalization is seen as yellow in canalicular networks of several parietal cells. F-actin staining continues into lining of gland lumen. |
| Blank spaces between narrow apical poles of parietal cells are not empty, but are occupied by other cells that secrete pepsinogen or mucus. They have too little ezrin or F-actin (except for F-actin at their apical membranes, which form most of the lining of the gland lumen) to be visible at the exposure settings used for parietal cells. |
| THE EMBO JOURNAL, Vol. 10, No. 9, 2363-2373, Sept. 1991 | |
The secretion-stimulated
|
| D. Hanzel, H. Reggio, A. Bretscher, J. G. Forte and P. Mangeat Department of Molecular and Cell Biology, University of California, Berkeley, CA 94720 |
From Duman, et al., J Cell Sci. 2002:
 |
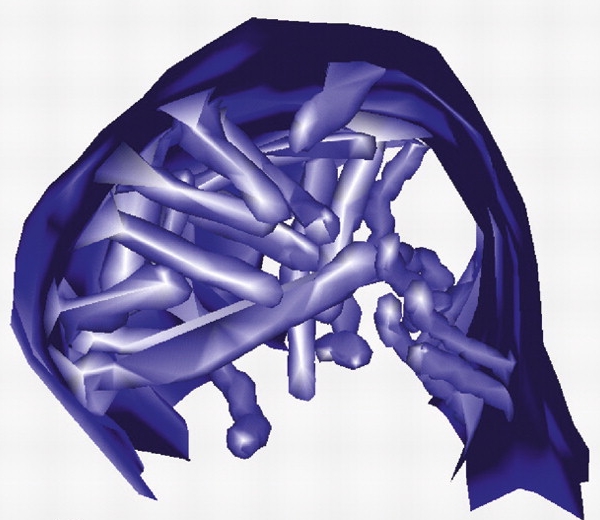 |
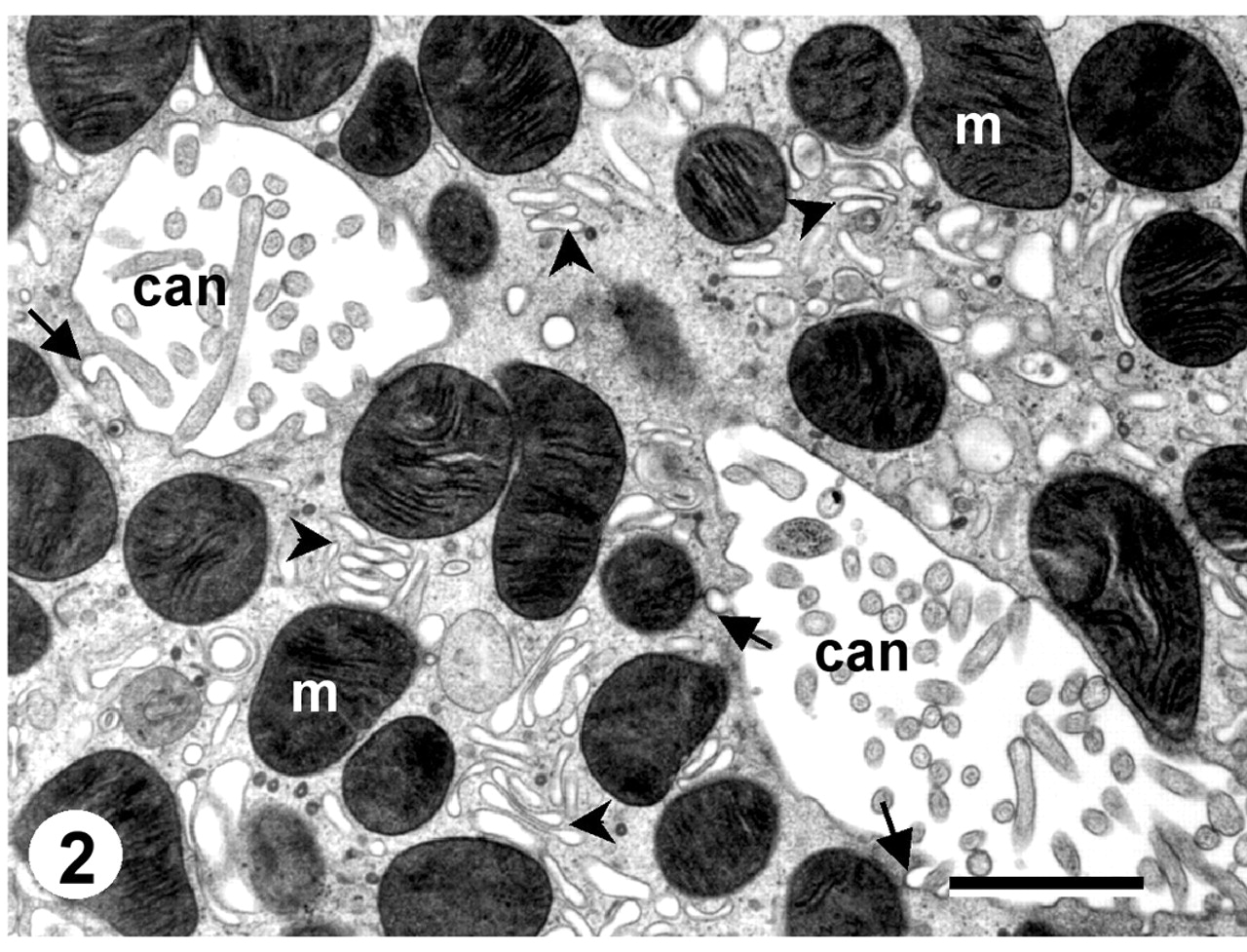
| TEM of canalicular cross-sections in gastric parietal cell. Many mitochondria (m) and some areas of tubulovesicles (arrowheads) surround the canaliculi. A few examples of endocytosis from the canalicular membrane (arrows) are also seen. Many microvilli extend into the canalicular lumina, most seen in cross-section, but a few lying in the plane of the thin section. Bar = 1μm. (Click to see large image in new window.) |
3-dimensional reconstruction of microvilli in portion of canaliculus, from serial ultra-thin sections of rabbit gastric glands |
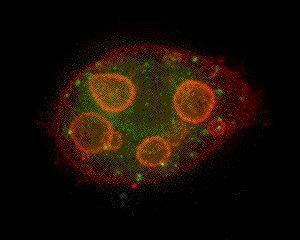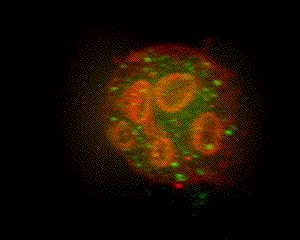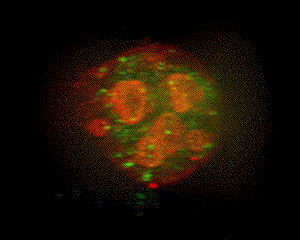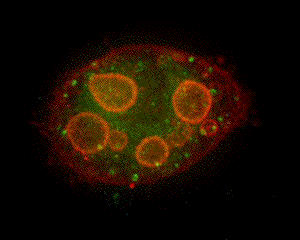 |
When parietal cells are isolated and cultured (method of Chew, et al., 1989 PubMed link), the apical pore closes and the previously basolateral membrane becomes the complete plasma membrane. The canaliculi pinch off from this plasma membrane to form several intracellular vacuoles that retain apical membrane characterisics. Stimulation causes tubulovesicles to fuse with these engulfed apical membranes. The accompanying graphic is a 3-D reconstruction from serial-section confocal microscopy of a cultured, resting parietal cell. It was probed with green-labeled anti-H,K-ATPase, identifying the tubulovesicles, and with red-labeled phalloidin, which binds to F-actin, concentrated at the vacuolar (apical) membranes and, more diffusely, near the plasma membrane. In this cell, small amounts of H,K-ATPase remaining at the apical membrane cause the vacuolar outlines to appear slightly orange compared to the pure red seen at the periphery of the cell. Such cultured cells are being used in our lab for expression of certain proteins altered by site mutation (e.g., of phosphorylation sites) and by linkage to Green Fluorescent Protein (GFP) to study their function and localization in parietal cell activation and determination of cell polarity. (See Am J Physiol Cell link, May 2010) |