Research
We are interested in understanding the cellular and molecular mechanisms underlying the development and maintenance of neuronal polarity, the formation and plasticity of synapses, and activity-dependent modification of neural circuits.
Current Research Interests
| Using cultured hippocampal neurons, we are examining the cytoplasmic events associated with axon/dendrite specification by intrinsic or extrinsic factors, and cytoplasmic trafficking mechanisms for maintaining neuronal polarity after axon/dendrite differentiation. In particular, we focus on early localized cytoplasmic signals triggered by extracellular polarizing factors, including gradients of secreted proteins and mechanical stress, that may induce a stable signal for axon initiation, and long-range inhibitory signals that prevent the formation of multiple axons. On the maintenance of neuronal polarity, we are examining the mechanisms underlying the sorting of axonal vs. somatodendritic components in the newly polarized neurons. Our current attention is focused on the recently discovered cytoplasmic F-actin-based “filter” for cytoplasmic components at the axonal initial segment- for example, how vesicular cargos driven by KIF motors are sorted by this filter for selective axonal entry. | |||
|
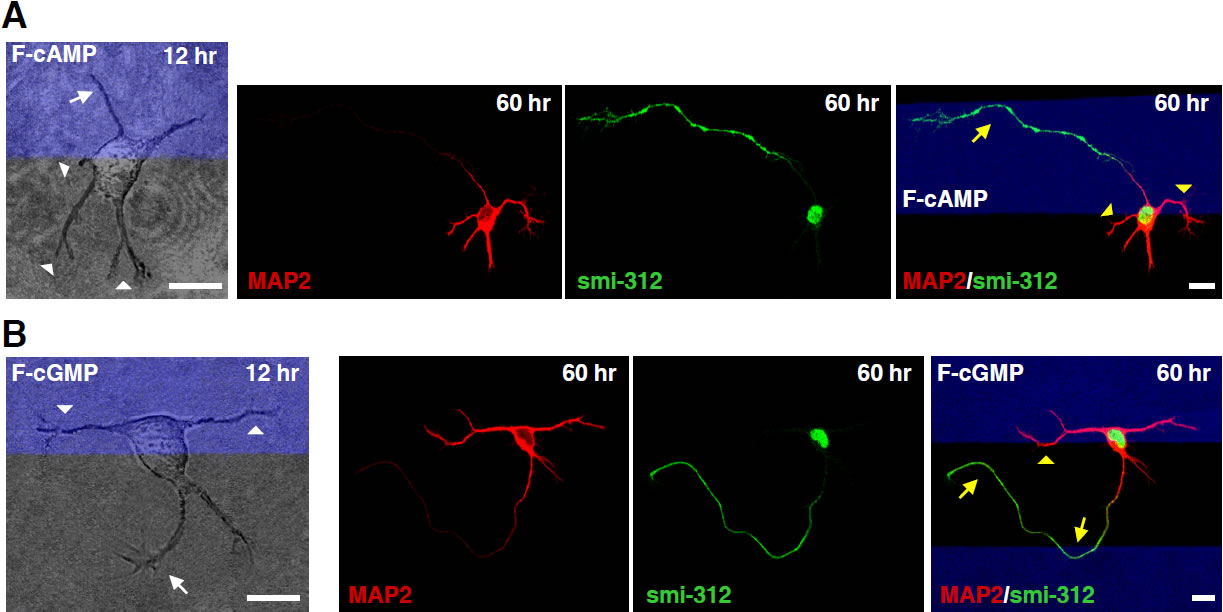
Antagonistic effects of cAMP and cGMP activities on the initiation of axons and dendrites. A and B, Images of hippocampal neurons cultured on substrates coated with stripes (blue) of membrane-permeant F-cAMP or F-cGMP, taken at 12 and 60 hr after cell plating and immunostaining (at 60 hr) for axons and dendrites with smi-312 and MAP2 antibodies, respectively. White arrows and arrowheads marked neurites that later became axons and dendrites, respectively. Yellow symbols marked turning of axons and dendrites at the stripe boundary. Scale, 10 μm. (From Selly et al., Science, 327:547-552, 2010)
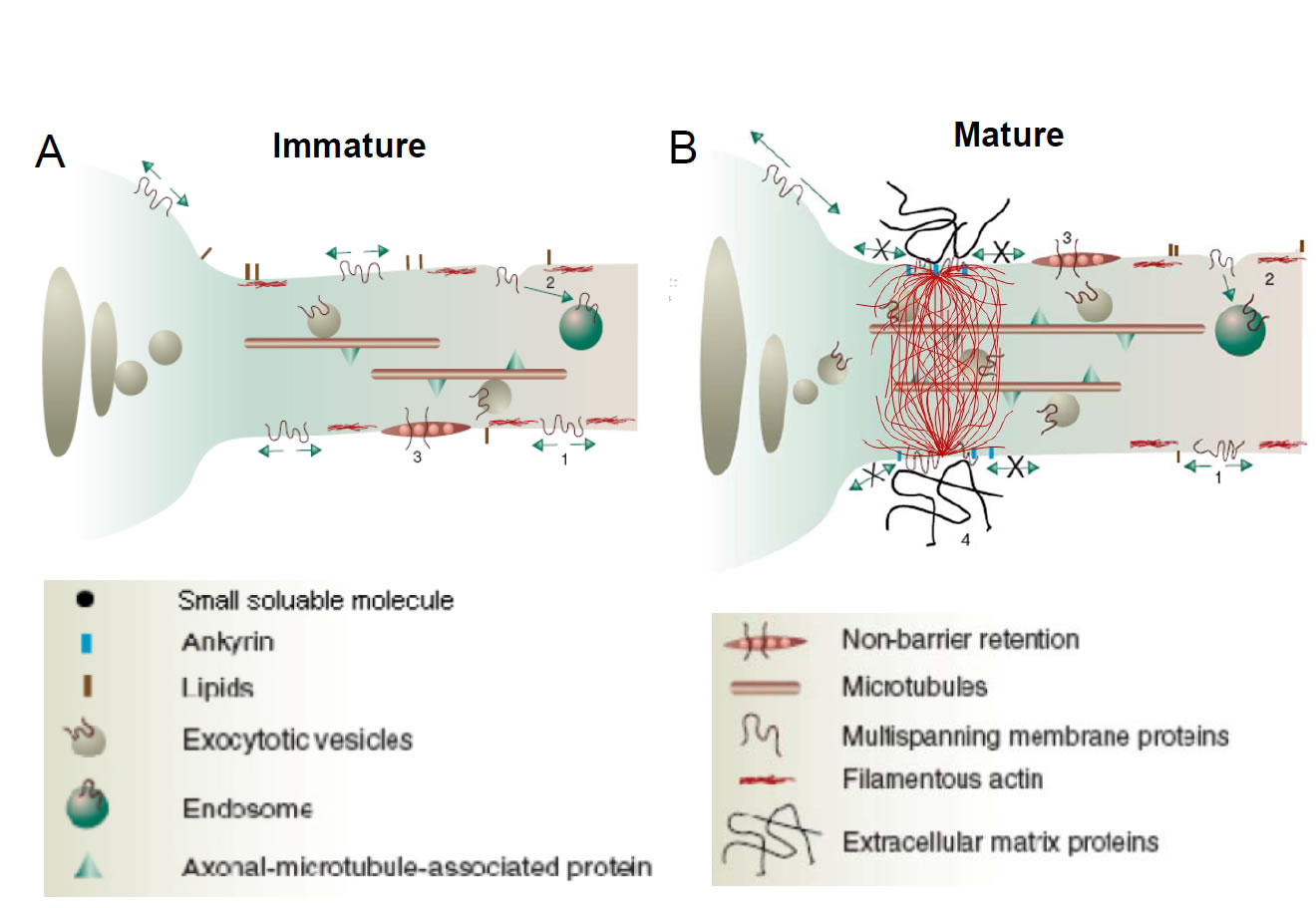
A Hypothesized Selective Filter for Cytoplasmic Transport at the Axon Initial Segment (Left) In newly polarized hippocampal neurons in 3-day old cultures, both axonal and dendritic vesicular carriers are transported into the growing axon. (Right) In more mature cultures (5-d or more), an ankyrin G- and F-actin-dependent structure emerges in the cytoplasm of the axon initial segment, imposing a selective filter for diffusion of large macromolecules and transport of vesicular carriers into the axon. Axonal entry was allowed for motor-cargo complex with higher transport efficacy. This selective filtering may contribute to preferential segregation of cellular components in polarized neurons.(For more information, please refer to Song et al.Cell,136:1148-1160, 2009) |
|||
Last Updated in Jan. 2010. Send feedback to Poo Lab
Early synaptic connections in the developing nervous system undergo substantial remodeling in response to electrical activity. Using rat brain slices in vitro and Xenopus/zebrafish retinotectal system in vivo, we are examining how various patterns of electrical activity and sensory inputs induce the strengthening or weakening of synaptic connections as well as the up- or down-regulation of intrinsic neuronal excitability, and how such activity-induced synaptic and neuronal modifications may participate in the developmental refinement of neural circuits and the emergence of integrative functions of neural circuits, e.g., specific receptive field properties of visual neurons.
Information in the nervous system may be coded by both the rate and timing of neuronal spikes. Recent findings of spike timing-dependent plasticity (STDP) at many synapses have fueled the interest on the role of spike timing in information processing and storage in the neural circuit. Induction of long-term potentiation (LTP) and long-term depression (LTD) in a variety of in vitro and in vivo systems depends on the temporal order of pre- and postsynaptic spiking. We have also shown spike-timing dependent bi-directional modifications of neuronal excitability and dendritic integration in cell cultures and brain slices. We are interested in understanding the role of STDP in activity-dependent processing and storage of information in neural circuits of the hippocampus, tectum, visual cortex, and ventral tegmental area of the midbrain.
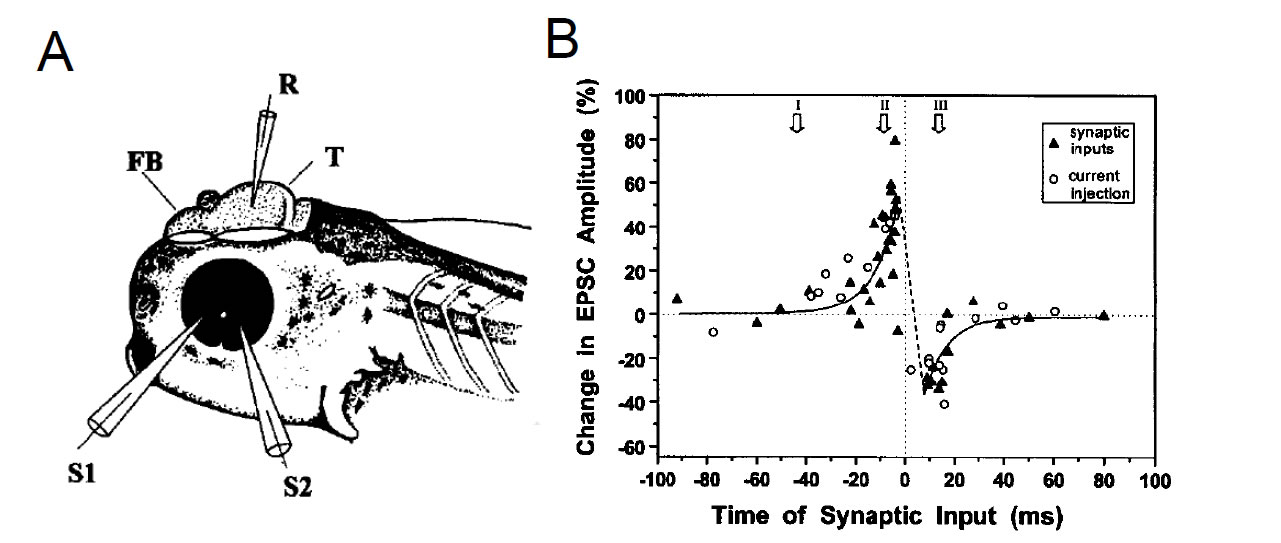
The critical window for spike-timing dependent synaptic potentiation and depression. In vivo whole-cell recording from Xenopus tadpole retinotectal neurons. Synaptic inputs activated repetitively within 20 ms before spiking of the tectal neuron become potentiated, whereas inputs activated within 20 ms after spiking become depressed. (From Zhang et al., Nature, 395: 37-44, 1998)
Neurotrophins as synaptic modulators
Based on the observations that neurotrophic factors can exert acute effects on neuronal morphology and functions, we are interested in the mechanism controlling neurotrophin secretion at the axon and dendrites, and whether and how activity-induced secretion of neurotrophins, e.g., brain-derived neurotrophic factor (BDNF), modulates synaptic efficacy and plasticity in various brain regions. Our recent attention is focused on the function of BDNF in modulating synaptic plasticity in the reward circuit, e.g., ventral tegmental area and medial prefrontal cortex of the rat, during the development of cocaine addiction and following withdrawal from cocaine exposure.
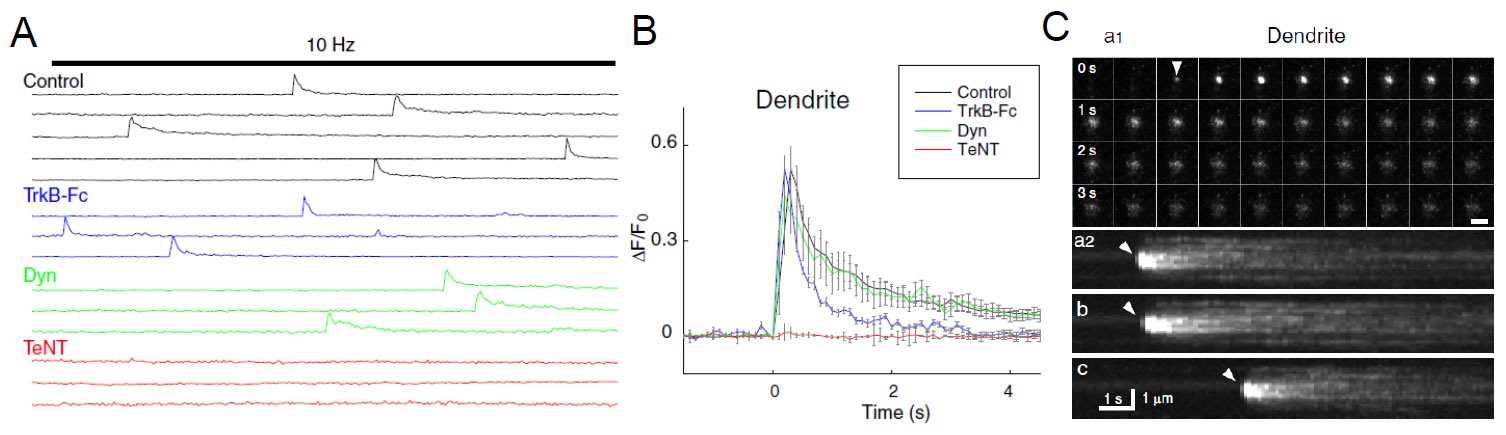
TIRF video recording of the fusion of single BDNF–pHluorin vesicles. A, Sample traces of BDNF–pHluorin fluorescence changes (ΔF ) measured at the center of each vesicle at dendrite of control neurons, neurons perfused with TrkB-Fc-containing solution (4 ng/ml), or neurons pretreated with dynasore (Dyn; 80 mM) or TeNT (10 nM). Each line represents a single vesicle. Bar indicates field stimulation at 10 Hz for 300 pulses. B, Average traces of BDNF–pHluorin fluorescence transients at dendrite as those shown inAand C, normalized by the basal fluorescence of each puncta (F0) and with the onset time of the transients aligned. C, Representative images of single vesicles at dendrite under 10 Hz (300 pulses) stimulation. a1, Consecutive images of a fusion event at the axon taken at 4 s interval. Scale bar, 2 μm. a2, Pseudo-line scan images for 12 s along the center of the spot for the vesicle shown in a1. b, c, Two other examples of vesicles undergoing fusion events. Repeated opening of the fusion pore was found for the vesicle shown in c. Arrowhead, Opening of fusion pore. See the movie (From Matsuda et al., J. Neurosci, 29: 14185-14198, 2009)