Precise modulation of neuronal activity with synthetic photoswitchable ligands
Abstract
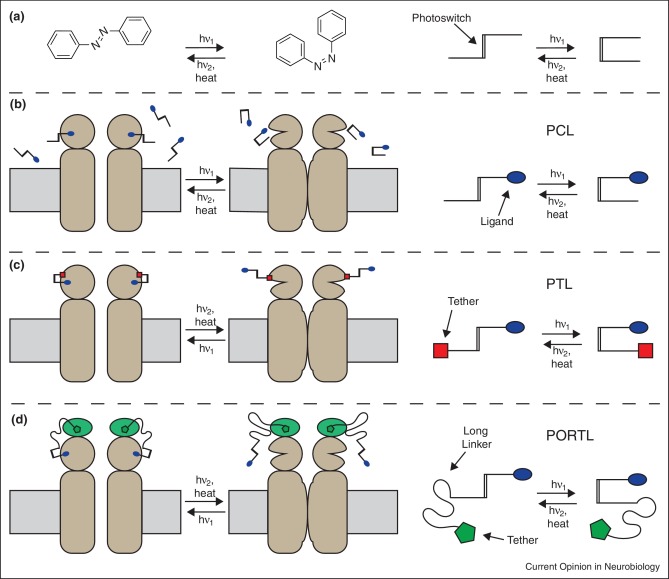
To tackle some of the most fundamental questions in neurobiology at the molecular level, classical genetics and pharmacology have been effective tools to perturb the activity of neuronal receptors and ion channels. However, conventional pharmacology has several drawbacks that are especially problematic when studying neural circuits and synapses. First, standard diffusion and partitioning of the ligand mean poor spatial and temporal control of ligand activity. Second, even in cases of high specificity for ligand action, it is relatively common for the receptor or channel of interest to be expressed on multiple nearby cell types, or even on both sides of a synapse, making it difficult to isolate the effects of the target receptor within the circuit or synapse of interest. Light-based techniques that operate at the intersection of chemistry, biology, and neuroscience have been developed to overcome these challenges. We focus here on systems that contain a synthetic photoswitch, a small molecule that absorbs light to reversibly change its shape. The most commonly used photoswitch in biological applications is azobenzene due to its synthetic tractability, tunable photochemical properties, and biological compatibility. The lowest energy isomer, the straight trans-azobenzene, isomerizes to the bent cis-azobenzene configuration upon irradiation with near-UV light. Subsequent irradiation with longer wavelength visible light, or thermal relaxation, leads the metastable cis-azobenzene to revert to the trans-azobenzene isomer.