Caenorhabditis elegans paraoxonase-like proteins control the functional expression of DEG/ENaC mechanosensory proteins
Abstract
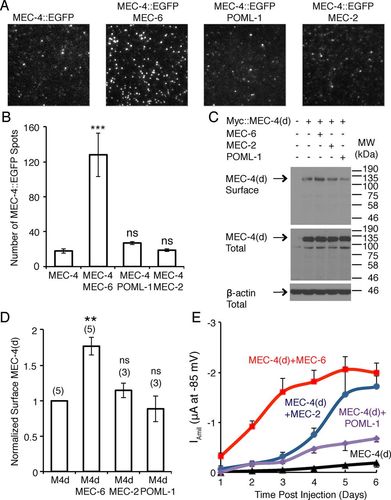
Caenorhabditis elegans senses gentle touch via a mechanotransduction channel formed from the DEG/ENaC proteins MEC-4 and MEC-10. An additional protein, the paraoxonase-like protein MEC-6, is essential for transduction, and previous work suggested that MEC-6 was part of the transduction complex. We found that MEC-6 and a similar protein, POML-1, reside primarily in the endoplasmic reticulum and do not colocalize with MEC-4 on the plasma membrane in vivo. As with MEC-6, POML-1 is needed for touch sensitivity, the neurodegeneration caused by the mec-4(d) mutation, and the expression and distribution of MEC-4 in vivo. Both proteins are likely needed for the proper folding or assembly of MEC-4 channels in vivo as measured by FRET. MEC-6 detectably increases the rate of MEC-4 accumulation on the Xenopus oocyte plasma membrane. These results suggest that MEC-6 and POML-1 interact with MEC-4 to facilitate expression and localization of MEC-4 on the cell surface. Thus MEC-6 and POML-1 act more like chaperones for MEC-4 than channel components.