Cooperative binding of stromal interaction molecule 1 (STIM1) to the N and C termini of calcium release-activated calcium modulator 1 (Orai1)
Abstract
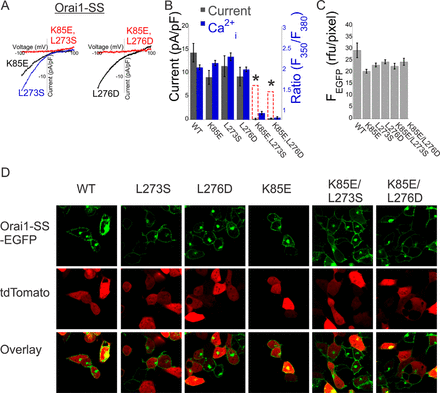
Calcium flux through store-operated calcium entry is a central regulator of intracellular calcium signaling. The two key components of the store-operated calcium release-activated calcium channel are the Ca(2+)-sensing protein stromal interaction molecule 1 (STIM1) and the channel pore-forming protein Orai1. During store-operated calcium entry activation, calcium depletion from the endoplasmic reticulum triggers a series of conformational changes in STIM1 that unmask a minimal Orai1-activating domain (CRAC activation region (CAD)). To gate Orai1 channels, the exposed STIM1-activating domain binds to two sites in Orai1, one in the N terminus and one in the C terminus. Whether the two sites operate as distinct binding domains or cooperate in CAD binding is unknown. In this study, we show that the N and C-terminal domains of Orai1 synergistically contribute to the interaction with STIM1 and couple STIM1 binding with channel gating and modulation of ion selectivity.