A spinal opsin controls early neural activity and drives a behavioral light response
Abstract
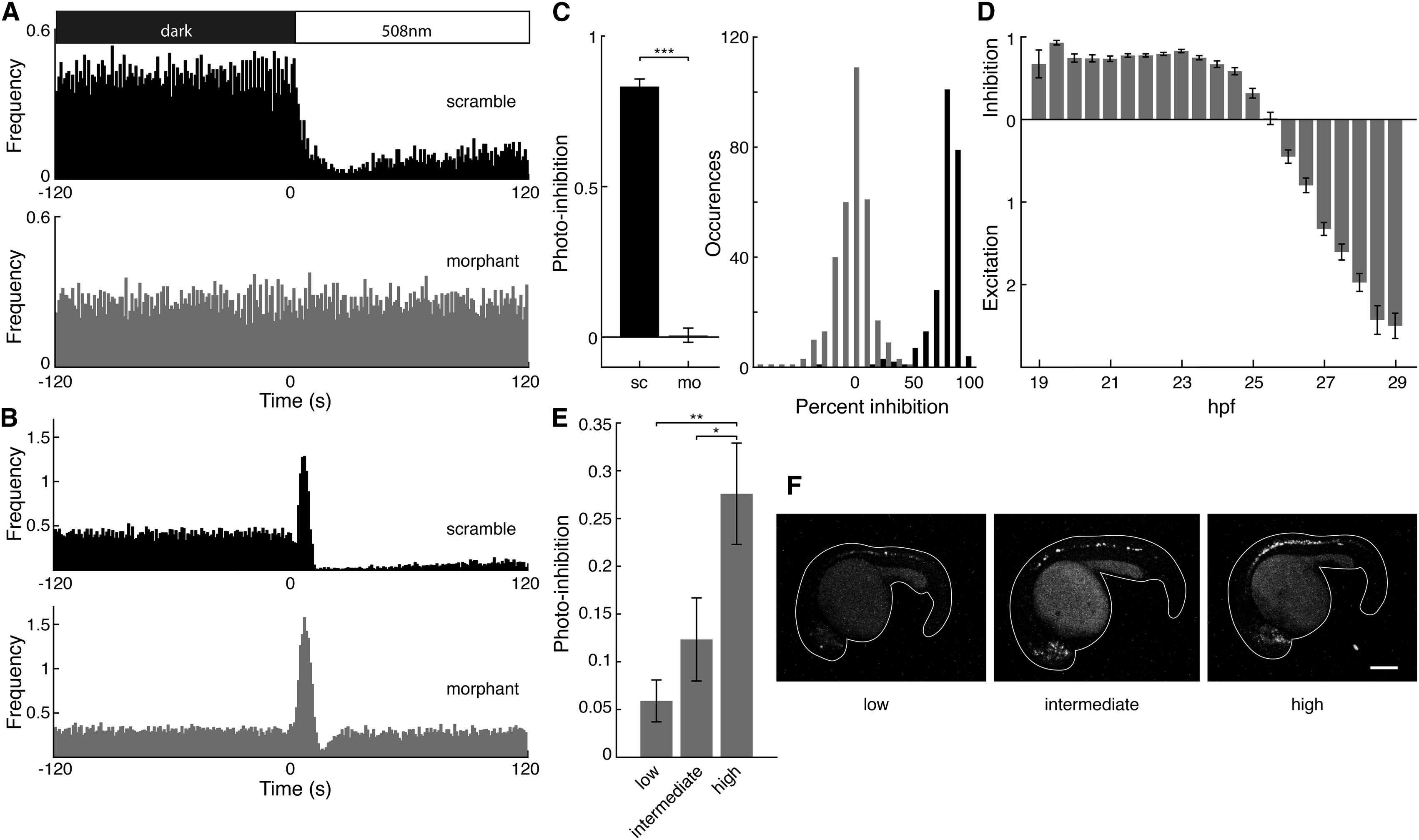
Nonvisual detection of light by the vertebrate hypothalamus, pineal, and retina is known to govern seasonal and circadian behaviors. However, the expression of opsins in multiple other brain structures suggests a more expansive repertoire for light regulation of physiology, behavior, and development. Translucent zebrafish embryos express extraretinal opsins early on, at a time when spontaneous activity in the developing CNS plays a role in neuronal maturation and circuit formation. Though the presence of extraretinal opsins is well documented, the function of direct photoreception by the CNS remains largely unknown. Here, we show that early activity in the zebrafish spinal central pattern generator (CPG) and the earliest locomotory behavior are dramatically inhibited by physiological levels of environmental light. We find that the photosensitivity of this circuit is conferred by vertebrate ancient long opsin A (VALopA), which we show to be a Gαi-coupled receptor that is expressed in the neurons of the spinal network. Sustained photoactivation of VALopA not only suppresses spontaneous activity but also alters the maturation of time-locked correlated network patterns. These results uncover a novel role for nonvisual opsins and a mechanism for environmental regulation of spontaneous motor behavior and neural activity in a circuit previously thought to be governed only by intrinsic developmental programs.