Phospholipase D2 specifically regulates TREK potassium channels via direct interaction and local production of phosphatidic acid
Abstract
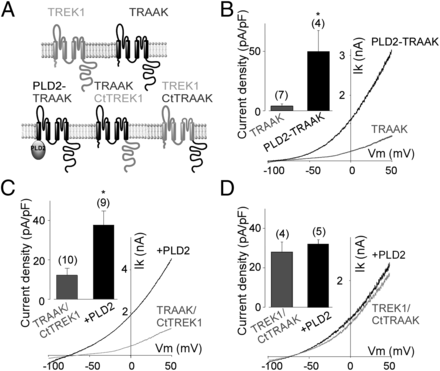
Our work provides evidence for a mechanism for the formation of membrane microdomains in which the local concentration of a phospholipid can change independently of the bulk membrane to confer selectivity on membrane protein regulation. We found that, despite the fact that all TWIK-related K channel (TREK) family members are sensitive to phosphatidic acid (PA), only TREK1 and TREK2 are potentiated by phospholipase D2 (PLD2) (which produces PA), but not by PLD1. This surprising specificity is due to the direct binding of PLD2 to TREK. This binding allows a local PA production that tonically activates the channel. Furthermore, we found the local signaling via PA to have a secondary focusing effect for primary alcohols, which inhibit the channel by altering the PA microdomain.