Photoswitching of cell surface receptors using tethered ligands
Abstract
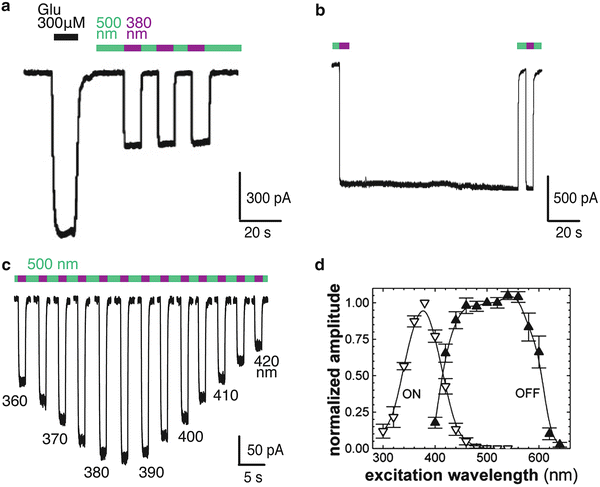
Optical probing and manipulation of cellular signaling has revolutionized biological studies ranging from isolated cells to intact tissues in the live animal. A promising avenue of optical manipulation is Chemical Optogenetics (or Optogenetic Pharmacology), an approach for engineering specific proteins to be rapidly and reversibly switched on and off with light. The approach employs synthetic photoswitched ligands, which can be reversibly photo-isomerized to toggle back and forth between two conformations in response to two wavelengths of light. We focus here on the photoswitched tethered ligand (PTL) approach in which the PTL is covalently attached in a site-directed manner to a signaling protein. For this a ligand anchoring site is introduced at a location which allows the ligand to dock only in one of the light-controlled conformations, thus enabling liganding to be rapidly switched. The ligand can be an agonist, antagonist or an active site (or pore) blocker. In principle, orthogonal chemistries of attachment would make PTL anchoring completely unique. However, extremely high specificity of remote control is also obtained by cysteine attachment because of the ligand specificity and precise geometric requirements for liganding. We describe here the design of light-gated ionotropic and metabotropic glutamate receptors, the selection of a site for cysteine placement, the method for PTL attachment, and a detailed protocol of photoswitching experiments in cultured cells. These descriptions can guide applications of Chemical Optogenetics to other receptors and serve as a starting point for use in more complex preparations.