Subunit organization and functional transitions in Ci-VSP
Kohout, S.C., Ulbrich, M.H., Bell, S.C., Isacoff, E.Y. 2007, Nature Structural & Molecular Biology
Abstract
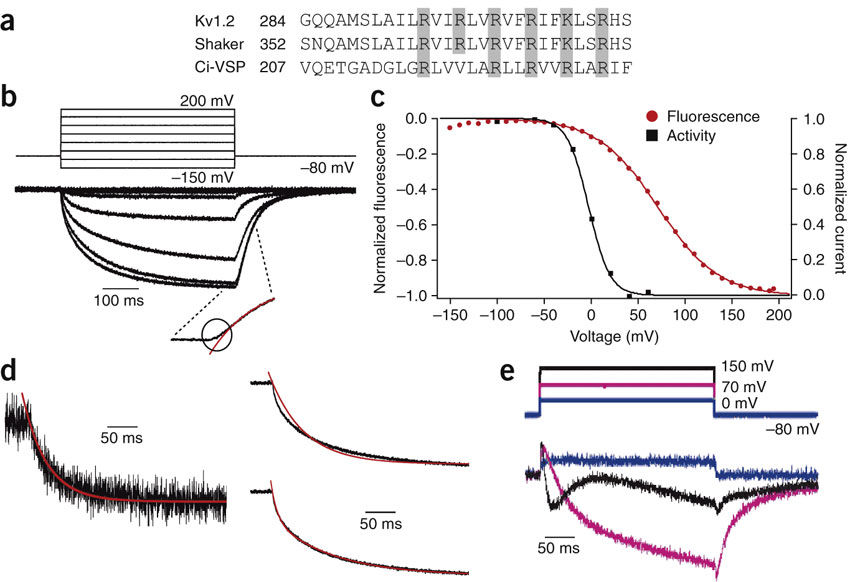
Voltage-sensing domains (VSDs) confer voltage dependence on effector domains of membrane proteins. Ion channels use four VSDs to control a gate in the pore domain, but in the recently discovered phosphatase Ci-VSP, the number of subunits has been unknown. Using single-molecule microscopy to count subunits and voltage clamp fluorometry to detect structural dynamics, we found Ci-VSP to be a monomer, which operates independently, but nevertheless undergoes multiple voltage-dependent conformational transitions.