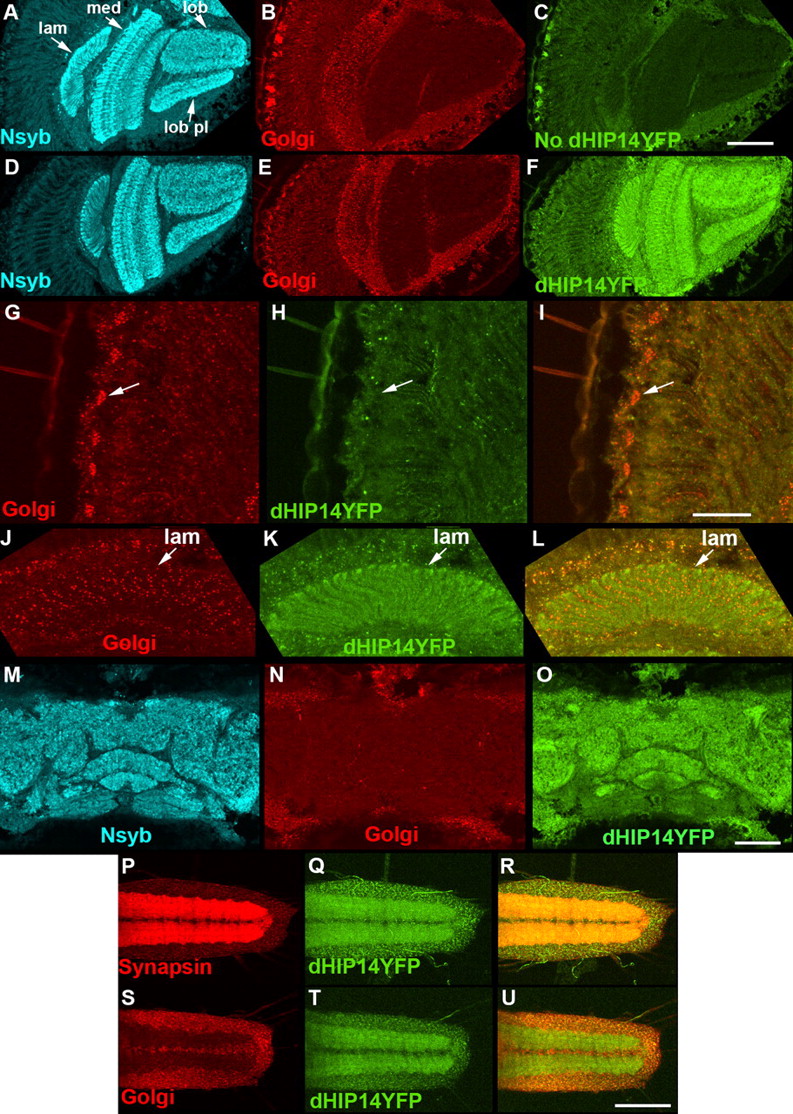
Drosophila huntingtin-interacting protein 14 is a presynaptic protein required for photoreceptor synaptic transmission and expression of the palmitoylated proteins synaptosome-associated protein 25 and cysteine string protein
Abstract
Membrane depolarization causes voltage-gated ion channels to transition from a resting/closed conformation to an activated/open conformation. We used voltage-clamp fluorometry to measure protein motion at specific regions of the Shaker Kv channel. This enabled us to construct new structural models of the resting/closed and activated/open states based on the Kv1.2 crystal structure using the Rosetta-Membrane method and molecular dynamics simulations. Our models account for the measured gating charge displacement and suggest a molecular mechanism of activation in which the primary voltage sensors, S4s, rotate by ∼180° as they move “outward” by 6–8 Å. A subsequent tilting motion of the S4s and the pore domain helices, S5s, of all four subunits induces a concerted movement of the channel’s S4-S5 linkers and S6 helices, allowing ion conduction. Our models are compatible with a wide body of data and resolve apparent contradictions that previously led to several distinct models of voltage sensing.