A fluorescent probe designed for studying protein conformational change
Abstract
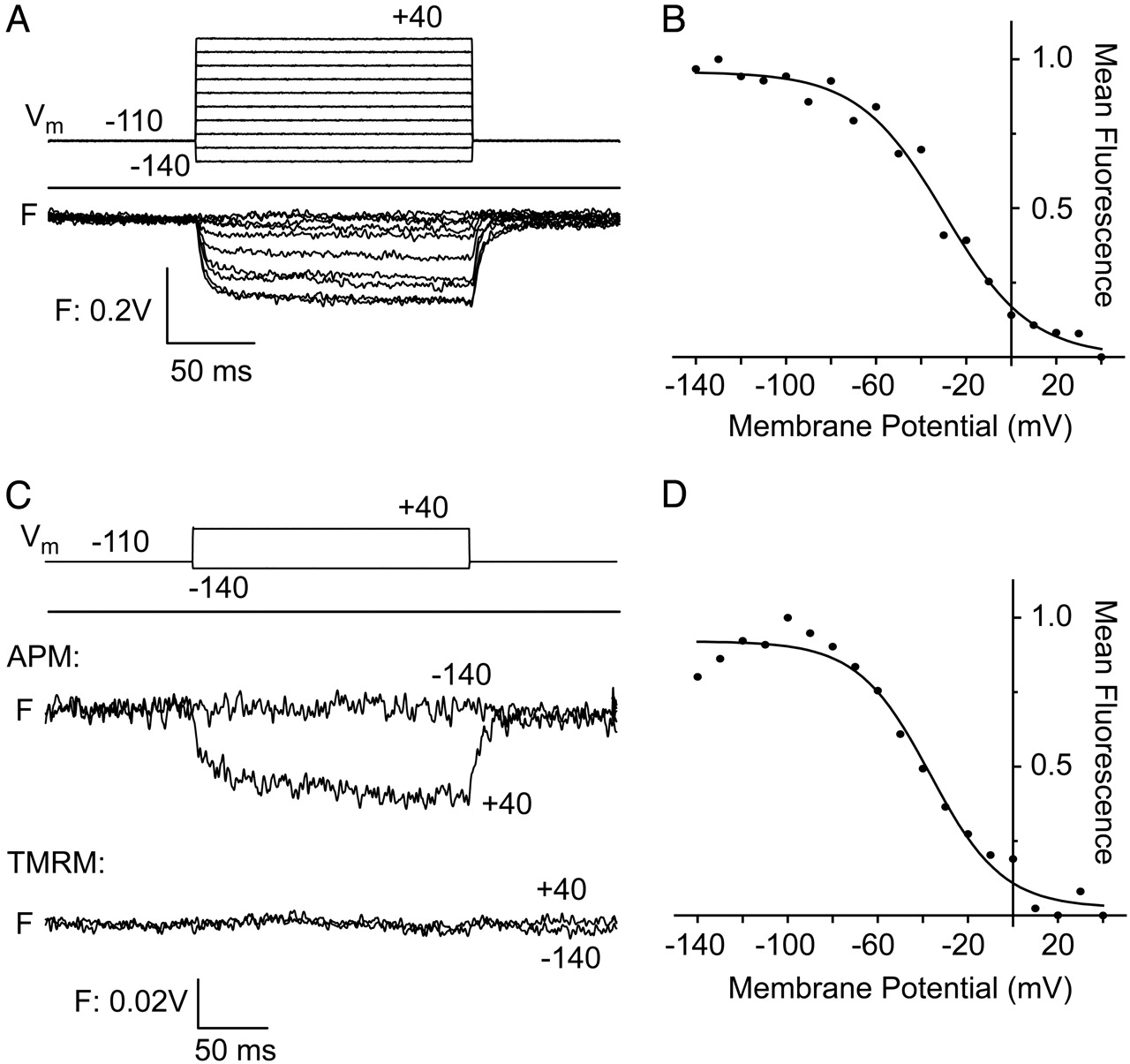
The usefulness of fluorescence in studying protein motions derives from its sensitivity, kinetic resolution, and compatibility with both live cells and physiological assays. Recent advances in microscopy and membrane protein purification have permitted the observation of fluorescence changes that accompany the functional transitions of complex eukaryotic membrane proteins. These techniques rely on probes that can clearly report the environmental changes of specific residues, but most commonly available side-chain-reactive probes are not well suited for this purpose. Here, we introduce a red Cys-reactive probe, aminophenoxazone maleimide (APM), designed with improved chemical and spectral properties for reporting protein conformational change. APM is compact, uncharged, and has a short linker between probe and protein, all of which ensure that it can closely track the motions of the side chain to which it is attached. It undergoes large polarity-dependent changes in Stokes shift, as well as large bathochromic shifts in both excitation maximum (from 521 nm in toluene to 598 nm in water) and emission maximum (580 nm to 633 nm). These polarity-dependent spectral changes offer a potentially simple means of relating fluorescence to local structure and motion, although they are partially offset by some complicating factors in APM fluorescence. We find that, like a rhodamine maleimide, APM senses the conformational changes underlying voltage sensing in the Shaker potassium channel, and it is superior at a site that shows limited reactivity to the rhodamine. The spectral characteristics of APM can also report subtle differences between aqueous positions in purified preparations of the beta2 adrenergic receptor.