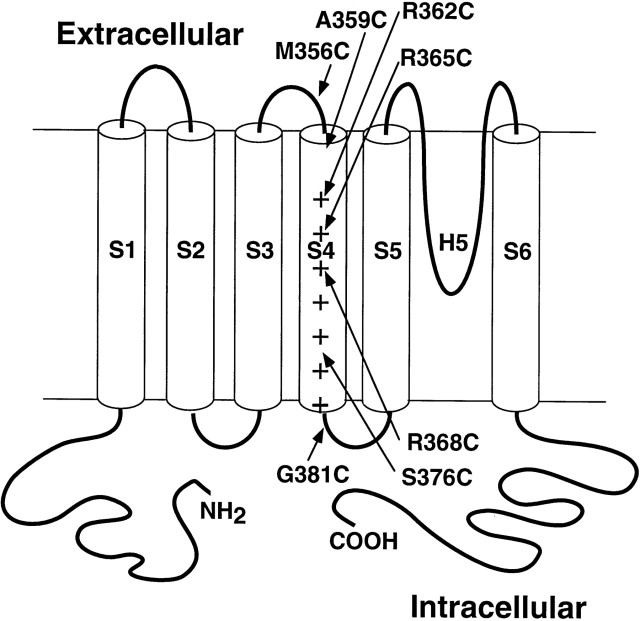
Transmembrane movement of the Shaker K+ channel S4
Abstract
We have probed internal and external accessibility of S4 residues to the membrane-impermeant thiol reagent methanethiosulfonate-ethyltrimethylammonium (MTSET) in both open and closed, cysteine-substituted Shaker K+ channels. Our results indicate that S4 traverses the membrane with no more than 5 amino acids in the closed state, and that the distribution of buried residues changes when channels open. This change argues for a displacement of S4 through the plane of the membrane in which an initially intracellular residue moves to within 3 amino acids of the extracellular solution. These results demonstrate that the putative voltage-sensing charges of S4 actually reside in the membrane and that they move outward when channels open. We consider constraints placed on channel structure by these results.