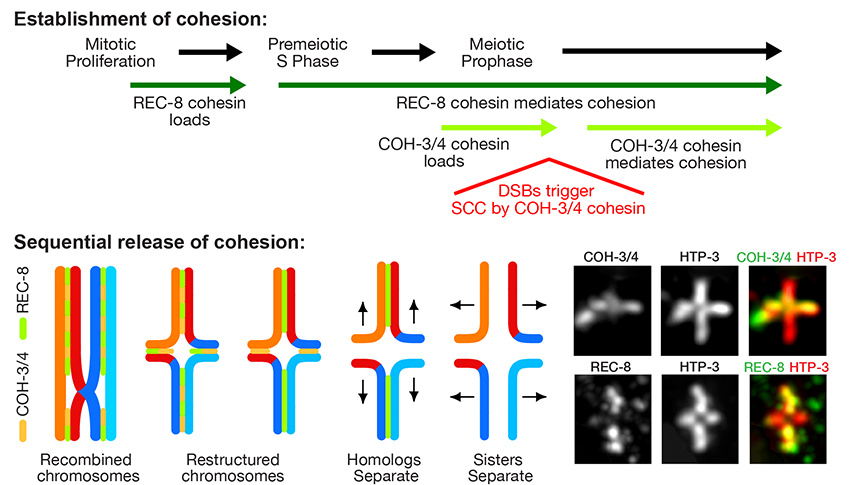
Figure 8: Divergent kleisin subunits of cohesin specify distinct mechanisms
that tether and release meiotic chromosomes. Establishment of cohesion (top).
REC-8 and COH-3/4 cohesins load onto chromosomes at different times, using shared
and separate loading factors, and establish sister chromatid cohesion by different
mechanisms. Cohesion mediated by REC-8 is established after pre-meiotic DNA
replication and before the onset of meiosis. In contrast, bound COH-3/4 cohesins require
the DNA double strand breaks made by the meiosis-specific SPO-11 endonuclease to
trigger cohesion. The sole other example of replication-independent cohesion
establishment occurs in mitotically proliferating yeast that suffer DNA damage.
Release of cohesion (bottom cartoon). In C. elegans, a single asymmetrically positioned
crossover forms between each pair of homologous chromosomes, and the site of the
crossover, rather than a centromere, determines where sister chromatid cohesion will be
released during the meiotic divisions. In the cartoon, sisters of one homolog are red and
orange, and sisters of the other are light and dark blue. After recombination is complete,
the chromosomes are restructured around the crossover to form a cruciform structure.
Just prior to the first meiotic division, SCC is released at the short arm to allow homologs
to separate. SCC persists at the long arm until just before the second meiotic division,
when it is released to allow sisters to separate.
REC-8 and COH-3/4 cohesins (represented schematically by orange and green ovals)
appear distributed all along sister chromatids in recombined homologous chromosomes
(see Figure 5) and in chromosomes restructured around the crossover.
Separase-independent removal of REC-8 and COH-3/4 cohesins from reciprocal
chromosomal territories then occurs in prometaphase, long before the first meiotic
division. Confocal micrographs of cruciform chromosomes stained with antibodies to the
axis protein HTP-3 and kleisin subunits REC-8 or COH-3/4 show the selective removal of
REC-8 cohesin from the short arms and its persistence along the long arms. The
reciprocal pattern occurs for COH-3/4 cohesins, removal from the long arm and
persistence on the short arm. The pattern of kleisin removal in prometaphase is
consistent with the phenotypes caused by null mutations. Loss of rec-8 causes all sisters
to separate prematurely in the first meiotic division. In contrast, loss of coh-3/4 causes
random segregation of homologous chromosomes. Our work suggests that COH-3/4
must be destroyed by separase to allow homologs to separate and then REC-8 must be
destroyed to allow sisters to separate.