

| The inability of CF airway cells to clear P. aeruginosa leads to the dramatic influx of neutrophils into the airways. This inflammatory response appears to be coordinated by the epithelial cells. The bacteria trigger the airway epithelia to secrete proinflammatory cytokines (such as interleukin-8). The bacterial factor(s) responsible for triggering the epithelial inflammatory response and the signaling pathway(s) activated remain a mystery. |

|
Hypothesis: P. aeruginosa secrete or release factors (e.g., lipopolysaccharide, LPS) that bind to receptors (e.g., toll-like receptors) that in turn activate MAPK and NFKB signaling pathways, resulting in the up- and down-regulation of families of genes involved in inflammation and facilitating the recruitment and transmigration of neutrophils.
| We are measuring gene expression in response to P. aeruginosa using two different methods. In collaboration with Steve Lory (Harvard) we have been performing gene microarray studies to determine the wide range of genes that are activated. Airway epithelial cells are grown to confluence, and P. aeruginosa are added selectively to the apical side. After different times, mRNA is extracted and gene microarray analysis is performed. Approximately 150 genes (out of about 5000) were activated by apical pa, and the patterns of gene expression indicate that the MAP kinase pathway and several different transcription factors that are commonly associated with inflammatory reactions are all upregulated. In addition, many genes associated with epithelial integrity (e.g., junctional proteins) are down regulated while those associated with neutrophil migration (IL-8, ICAM's and integrins) are upregulated. These studies will be continued using the Functional Genomics Center here at UC-Berkeley. |

|
 |
In addition, we are setting up a fluorescence method for measuring P. aeruginosa-induced IL-8 gene expression in single living airway epithelial cells. This method has been pioneered by Tsien and his colleagues (__), and we are using the same approach to determine the time course, cell specificity and the receptors and second messengers involved in the P. aeruginosa activation of IL-8 gene expression. The combined use of the microarray and the fluorescence assays will allow us great flexibility in determining the receptors, second messenger pathways and specific genes activated during the presence of apical P. aeruginosa in the airways of CF individuals. We will also study any differences in signalling between CF and wt epithelia. |
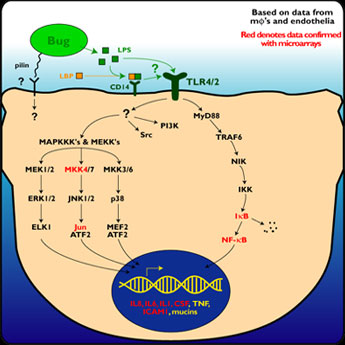
Hypothesis: P. aeruginosa (using the bacterial protein pilin) bind to receptors (asialo-GM1) on the epithelial cells that then trigger the second messenger Ca+2 and upregulation of the transcription factor NFAT to activate the IL-8 gene.
Normal and CF airway epithelial cells are grown to confluence on filters, filled with the Ca-sensitive dye fura-2 and mounted on the stage of a digital imaging microscope for measurement of cytosolic [Ca+2]. Our approach (see Sjaastad et al, 199_) allows us to recreate the physiological condition and add P. aeruginosa and also to perfuse different solutions onto the apical and basolateral sides selectively. We have found that P. aeruginosa bind only to basolateral membrane sites (Lee et al, 2000) of confluent airway epithelia, and that apical bacteria have no effect on airway epithelial cell [Ca+2]. Basolateral addition of P. aeruginosa leads to increases in [Ca+2], but only if the PA strains are cytotoxic. Use of mutant strains of P. aeruginosa and different perfusing solutions allowed us to conclude that cytotoxic (but not noncytotoxic) P. aeruginosa elicit increases in airway epithelial Cai by using pili to bind to the basolateral membranes and then secreting exotoxin U into the epithelial cells; this causes, after a delay of 30-50 mins, an inhibition of Ca+2 pumps in the endoplasmic reticulum and activation of store-activated Ca+2 channels in the basolateral membranes of the epithelial cells, leading to increases in cell [Ca+2] that precede, but are not required for, cell killing. Because apical P. aeruginosa do not activate airway epithelial cell [Ca+2], we conclude that in the physiological condition P. aeruginosa binding and Ca+2 signaling is irrelevant for activating IL-8 or other genes. We have now begun to investigate the role of Ca+2 in the P. aeruginosa-induced cytotoxicity.