


Photo credit: Mark Joseph Hanson
Ask any Berkeley faculty member why they came to Cal, and their answer is likely to include two factors: the strong spirit of collaboration and the high caliber of our graduate students. Each of these played a critical role in the exciting recent research discovery to come from the labs of Dirk Hockemeyer and Xavier Darzacq, whose study has deepened our understanding of cancer cell immortality.
A unifying feature of cancer cells is their unlimited capacity to replicate, and this cellular immortality is almost always reliant on the enzyme telomerase. Telomerase can elongate the caps, or telomeres, at the ends of chromosomes. In cells without telomerase, telomeres gradually break down with each cell division, limiting their replicative capacity. In the 1980s, the discovery of telomerase — an enzyme that can replenish the chromosome caps — was made by Berkeley’s Elizabeth Blackburn and Carol Greider with Harvard’s John Szostak, earning them the 2009 Nobel Prize in Physiology or Medicine.
Now the research emerging from Hockemeyer’s and Darzacq’s labs indicates that the way cancer cells acquire telomerase expression — and thereby immortality — is a more complicated process than previously believed.

Photo credit: Mark Joseph Hanson
As Hockemeyer was just establishing his lab as a new PI, he started working on the question of how mutations in the telomerase gene, which are frequently found in cancers, promote the disease. This problem attracted Kunitoshi Chiba, a PhD student from Japan. “We asked, ‘What happens to a normal cell that acquires a cancer-associated mutation in the telomerase gene?’” says Hockemeyer. “Is the mutation automatically making cells immortal?”
Chiba used CAS9-mediated genome editing to engineer human cells to carry these mutations. “We looked into what these mutations do in a normal cell — and that’s where we got a surprise,” he notes. “It turned out that these mutations were not enough to stop telomere shortening and to make cells immediately immortal.”
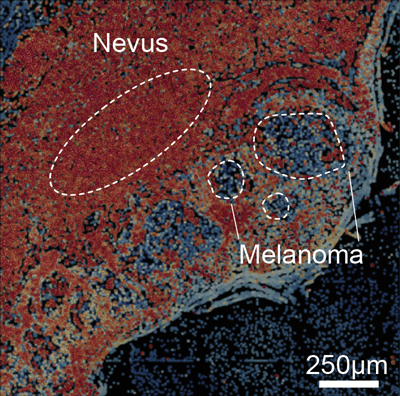
Next, two key collaborations pushed this project forward. First, Hockemeyer’s lab neighbor and fellow faculty researcher, Xavier Darzacq, mentioned a “structured illumination” microscope, which at the time was still just a prototype developed by the Santa Clara startup Optical Biosystems. Hockemeyer and Darzacq immediately recognized that this piece of equipment could be used to quantitatively, and with high-throughput, measure telomere length in large areas of a human tissue section.
Simultaneously, another of Hockemeyer’s graduate students, Franziska Lorbeer, attended a conference where she met Hunter Shain, a postdoc working at UCSF, and Boris Bastian, a leader in melanoma research. Shain’s work had previously demonstrated that in melanoma the telomerase mutations could occur early during cancer development. Using the new imaging technology, Lorbeer and Shain directly assessed telomere length changes in actual tumors.
Darzacq and his graduate student, David McSwiggen, then became the masterminds behind the project’s quantitative analysis of the melanoma imaging data. These experiments showed that, again, telomeres can shorten in cells with the telomerase mutation, highlighting that telomere length dynamics are a key determinant for the early stages of melanoma development.
Such discovery relies on collaboration at all levels. “Empowering students with this diversity of tools and ideas can only be done here at Berkeley,” Darzacq says. “It’s the most open and collaborative place I’ve ever seen.”
(Image by Dirk Hockemeyer/UC Berkeley and Boris Bastian/UCSF)




