A genetically encoded nanoparticle to elucidate the regulation of subcellular diffusion
During normal cell physiology, global effects such as mechanical, osmotic and pH changes will impact thousands of interactions in the cell. We are investigating the biophysical consequences of these perturbations. We hypothesize that modulation of the biophysical properties of the cytosol and nucleoplasm may be a widespread regulatory mechanism.
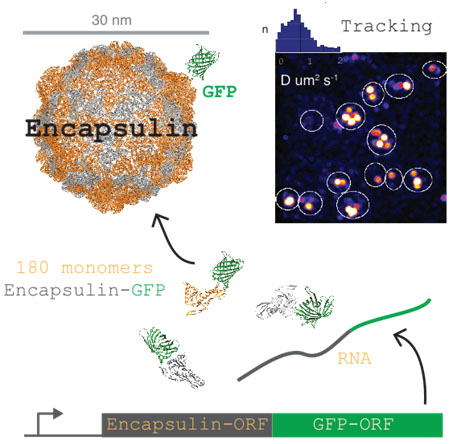
To enable the investigation of this hypothesis, we have developed a genetically encoded fluorescent nanoparticle as a reporter for cellular diffusion. This nanoparticle consists of an encapsulin fused to the GFP variant T-Sapphire. 180 encapsulin monomers self-assemble into a bright, stable particle of 30 nm diameter (Figure z ). Importantly, this large particle size samples length-scales at which anomalous diffusion becomes pronounced. We can now track hundreds of these particles in many cells simultaneously. This approach overcomes many of the technical difficulties that have hampered the efficient study of intracellular diffusion in the past.
|