Robert Saxton

Assistant Professor of Immunology and Molecular Medicine*
*and Chemistry
Research Interests
Although inflammatory immune responses are essential for protecting organisms against infection and disease, excessive inflammation can damage bystander tissues and compromise biological function. To prevent this, mammals have evolved a complex array of signaling molecules and receptors that ensure the protection and repair of host tissues. However, failure of these homeostatic systems can result in chronic inflammation, autoimmunity, and cancer — diseases with rapidly increasing prevalence in modern society.
The Saxton lab studies the mechanisms of cell communication that control tissue inflammation, repair, and homeostasis, with the goal of developing novel therapeutics to modulate these pathways in disease.
Our multidisciplinary research program uses a range of biochemical and structural biology techniques, together with cutting edge approaches in protein engineering, receptor pharmacology, and mouse models of inflammation in order to understand and control inflammatory signaling at the atomic, cellular, and organismal levels.
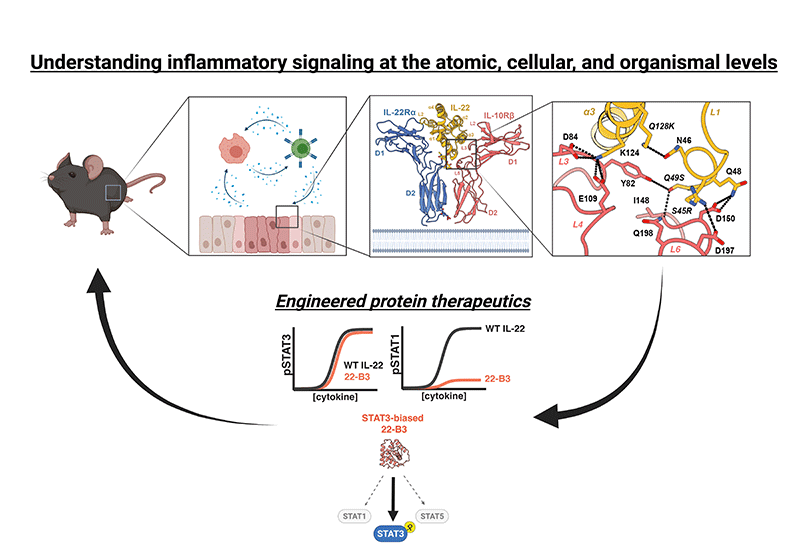
Current Projects
1. Resolution of inflammation
The resolution of inflammation is essential for returning to homeostasis following tissue damage, and persistent, unresolved inflammation is a central feature of nearly all human diseases. We seek to understand the signaling mechanisms that enable the efficient resolution of inflammation in order exploit these processes therapeutically. Our previous work has focused on the key anti-inflammatory cytokine interleukin-10 (IL-10), where we have used the cryo-EM structure of the IL-10 receptor complex to rationally design cell type selective IL-10 variants with enhanced therapeutic properties. These molecules are currently being developed for clinical use in autoimmune and chronic inflammatory disease.
2. Mechanisms of immune-mediated tissue repair
In addition to eliminating infectious agents, cells of the immune system also play key roles in repairing tissues following damage caused by either pathogens or the immune system itself. This role is particularly critical at epithelial barriers of the GI tract, skin, and lungs, which are constantly exposed to microbes and environmental damage. By understanding the mechanisms of immune-epithelial crosstalk that promote tissue repair, we hope to develop novel protein therapeutics that drive repair and promote mucosal healing in the context of diseases including IBD, psoriasis, and GvHD.
3. Metabolic signals that modulate immune function
Metabolic dysfunction such as in obesity or type 2 diabetes strongly predisposes for chronic inflammatory disorders and several types of cancer. Although it is clear that many aspects of immune function are regulated by metabolic signals (e.g. hormones, metabolites), the molecular mechanisms through which these signals modulate inflammation remain poorly understood. By studying the ligand-receptor systems that mediate metabolic-immune crosstalk we hope to identify new routes of controlling and preventing diseases associated with metabolic dysfunction.
Selected Publications
1. Saxton, R. A., Henneberg, L. T., Calafiore, M., Su, L., Jude, K. M., Hanash, A. M., & Garcia, K. C. (2021). The tissue protective functions of interleukin-22 can be decoupled from pro-inflammatory actions through structure-based design. Immunity, 54(4), 660–672.e9. https://doi.org/10.1016/j.immuni.2021.03.008
2. Saxton, R. A., Tsutsumi, N., Su, L. L., Abhiraman, G. C., Mohan, K., Henneberg, L. T., Aduri, N. G., Gati, C., & Garcia, K. C. (2021). Structure-based decoupling of the pro- and anti-inflammatory functions of interleukin-10. Science, 371(6535), eabc8433. https://doi.org/10.1126/science.abc8433
3. Saxton, R. A., Chantranupong, L., Knockenhauer, K. E., Schwartz, T. U., & Sabatini, D. M. (2016). Mechanism of arginine sensing by CASTOR1 upstream of mTORC1. Nature, 536(7615), 229–233. https://doi.org/10.1038/nature19079
4. Saxton, R. A., Knockenhauer, K. E., Wolfson, R. L., Chantranupong, L., Pacold, M. E., Wang, T., Schwartz, T. U., & Sabatini, D. M. (2016). Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science, 351(6268), 53–58. https://doi.org/10.1126/science.aad2087
Last Updated 2022-09-01